CYCLAZODONE POWDER
$59.99 – $259.99
Cyclazodone is sold for laboratory research use only. Terms of sale apply. Not for human consumption, nor medical, veterinary, or household uses. Please familiarize yourself with our Terms & Conditions prior to ordering.
- Description
- Additional information
Description
Cyclazodone Nootropic Powder
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
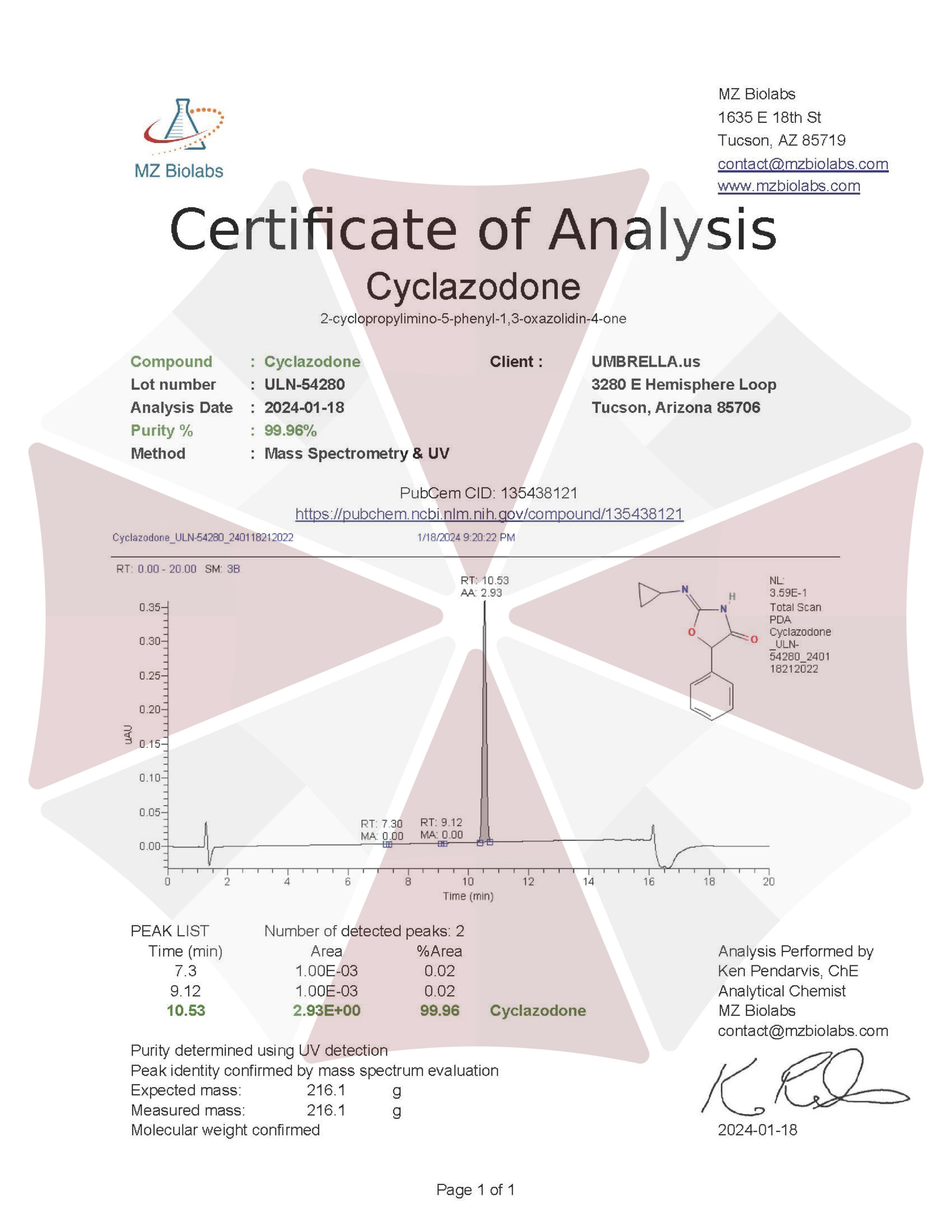
| CAS Number | 14461-91-7 |
| Other Names | Ciclazodona |
| IUPAC Name | 5-phenyl-1,3-oxazolidin-4-one |
| Molecular Formula | C₁₂H₁₂N₂O₂ |
| Molecular Weight | 216.24 |
| Purity | ≥99% Pure (LC-MS) |
| Liquid Availability | |
| Powder Availability | |
| Gel Availability | N/A |
| Storage | Store in cool dry environment, away from direct sunlight. |
| Terms | All products are for laboratory developmental research USE ONLY. Products are not for human consumption. |
What is Cyclazodone?
Cyclazodone was first developed by the American Cyanamid Company in the 1960s, and while research is limited on the effects of the nootropic it has been reported to enhance focus and stamina, promote mild euphoria, and trigger the release of dopamine and norepinephrine in the brain.
Nootropic compounds are best known for their cognitive enhancing abilities in individuals suffering from disorders such as Alzheimer’s diseases, schizophrenia, attention deficit hyperactivity disorder, or general age-related decline. Nootropics were initially developed in the 1960s with the synthesis of piracetam, an increasingly popular and highly researched compound. Since then, several highly researched nootropics such as phenylpiracetam hydrazide and aniracetam have been structurally derived from piracetam. Nootropics are named based on both their effects and their nature; the group of compounds is divided into four categories: classical nootropic compounds, cholinergic, plants and their extracts, and substances that increase brain metabolism. Each of these categories is under review while a substantial amount of research is being conducted examining uses of the compounds, indications and contraindications, experimental treatments, and potential effective dosages [1].
Main Research Findings
1) Treatment was shown to be moderately effective in adults with ADHD. While treatment was generally well-tolerated the researchers concluded that it should be considered as a secondary ADHD medication due to concerns of hepatic dysfunction.
2) When examining the effects of cyclazodone treatment in adolescents experiencing substance abuse disorder (SUD), the research team determined that the compound was capable of treating ADHD, but had minimal effects on conduct disorder (CD) or SUD.
3) Cyclazodone was found to be an effective treatment for attention deficit hyperactivity disorder (ADHD) in adolescents of an average age of 14 years old.
Selected Data
1) Nootropic compounds are known for their ability to improve cognitive functioning in cases related to attention deficit hyperactivity disorder (ADHD). Despite the increased awareness surrounding the mechanism behind ADHD in adults, there is limited research available regarding pharmaceutical interventions for the disorder. Cyclazodone is a derivative of the popular nootropic, cyclazodone, that is currently being investigated for the benefits the compound has the potential to elicit in individuals experiencing symptoms of ADHD [2].
The research team of Wilens et. al examined how administration of cyclazodone to adults with ADHD affected symptoms, as well as how the compound was tolerated by the test subjects. 35 participants exhibiting symptoms related to DSM-III-R and -IV ADHD took part in a 10-week, double-blind, crossover, placebo-controlled study where each subject received either a 3 mg/kg dose of cyclazodone, or a placebo equivalent.
Prior to the initiation of the experiment, standardized psychiatric instruments were used to diagnose participants with ADHD and take note of the manifestation of symptoms related to generalized depression and anxiety disorders. Each participant was required to attend biweekly follow up appointments where all symptoms of ADHD, depression, and anxiety were separately assessed in order to record instances of improvement or regression. Changes in ADHD symptoms were specifically measured using the ADHD symptom checklist and the Clinical global Impression scales of Severity and Improvement [2].
2) Data show that 75.% of high school students report using addictive substances while 11.4% of 13-18 year-old individuals experience a lifetime prevalence of SUD and an 8.3% 12-month prevalence of SUD. That being said, one of the primary comorbidities associated with adolescents experiencing a greater severity of SUD is ADHD [3].
Researchers Riggs et. al identified a gap in knowledge regarding the efficacy of cyclazodone administration in adolescents with ADHD and SUD, considering that current research typically focuses on the compound’s effects in young children or adults. A placebo-controlled randomized experiment was conducted in order to evaluate both the safety and efficacy measures of cyclazodone in adolescents with SUD, ADHD, and conduct disorder (CD) [4].
The experiment included 69 test subjects aged 13 to 19 experiencing symptoms of CD, SUD, and ADHD. All participants recruited for the study were randomly assigned to a treatment group receiving either an active dose of cyclazodone or a placebo compound. Over a 12-week experimental period all subjects received doses of either cyclazodone or a placebo compound ranging from 75 mg to 112.5 mg as tolerated by the participant. The doses were titrated over the course of four weeks until each test subject received a single dose of the compound every morning [4].
3) The research team of Riggs et. al conducted a similar study examining the effects of cyclazodone on comorbid ADHD in adolescents with both conduct disorder and substance use disorder. ADHD in these individuals can contribute to antisocial behaviors and continued substance use disorders, however, there is very little research regarding how treating the symptoms of ADHD, either with traditional or alternative methods, can contribute to the stabilization of substance use and behavioral disorders. Therefore, the purpose of this study was to not only reduce symptoms of ADHD, but to also gather information regarding the safety and tolerability of cyclazodone throughout this population of test subjects [5].
The study utilized 13 adolescent male test subjects residing in a residential substance use treatment program and exhibiting symptoms of CD, SUD, and ADHD. The Conners Hyperactivity Index, continuous performance tasks, and physical activity measurements were used to measure reduction of symptoms in the participants. All scores were obtained at baseline and after one month of treatment with cyclazodone. The maximum dosage administered to each patient ranged from 1.2 mg/kg to 3.3 mg/kg per day. After approximately one week of receiving the maximum dose of cyclazodone each subject was required to undergo a post medication assessment to record changes in symptoms [5].
4) As it was previously mentioned, nootropics such as cyclazodone and its parent compound cyclazodone have been shown to effectively treat ADHD in children and adults, but have not been sufficiently studied in adolescents. The research team of Bostic et. al emphasized the importance of studying alternative ADHD treatments for adolescents that go beyond the use of traditional central nervous system stimulants. The experimental procedure recruited twenty-one adolescents of an average age of 14 years old, to participate in a 10-week crossover design, double-blind study [6].
Each test subject was randomly assigned to receive either an active dose of cyclazodone or a placebo compound. The goal was to administer up to 3 mg/kg per day of the assigned compound; the dosing schedule was optimized by administering the compounds in one or two doses each day. The participants of the study were assessed at weekly follow-up appointments in order to record any instances of side effects or symptoms of ADHD, depression, anxiety, and oppositional defiant disorder (ODD). Changes in ADHD symptoms specifically were measured via the ADHD rating scale [6].
Discussion
1) The study conducted by Wilens et. al examined the effect of cyclazodone in 35 adults diagnosed with ADHD. Out of the 35 initially selected participants, 77% (27 individuals) completed the experimental protocol. While the goal of the researchers was to administer 3 mg/kg per day of cyclazodone or a placebo compound, findings from the final 4 weeks of the treatment period reported that a significantly lower dose of 2.2 mg/kg per day was best tolerated by the test subjects. Overall, the data collected indicated that treatment with cyclazodone resulted in significant reductions of ADHD symptoms when compared to the placebo compound [2].
The effectiveness of treatment was based on a predetermined rate of a 30% decrease in symptoms of ADHD. Out of the subjects treated with an active dose of cyclazodone, 50% reported an improvement in their symptoms. This was compared to the placebo group where only 17% of the test subjects reported an improvement in their symptoms. The reported results of this experiment indicate that treatment with cyclazodone Cyclazodone was first developed by the American Cyanamid Company in the 1960s, and while research is limited on the effects of the nootropic it has been reported to enhance focus and stamina, promote mild euphoria, and trigger the re is moderately effective in adults experiencing symptoms of ADHD.
In regards to the safety and overall tolerability of the compound, most test subjects responded best to a moderate dose of cyclazodone Cyclazodone was first developed by the American Cyanamid Company in the 1960s, and while research is limited on the effects of the nootropic it has been reported to enhance focus and stamina, promote mild euphoria, and trigger the re ranging from 120-160 mg per day, despite the original target dose of 3 mg/kg per day. The research team thought it was important to note that cyclazodone Cyclazodone was first developed by the American Cyanamid Company in the 1960s, and while research is limited on the effects of the nootropic it has been reported to enhance focus and stamina, promote mild euphoria, and trigger the re can be linked to hepatic dysfunction and therefore should be considered as a secondary treatment for ADHD considering its moderate levels of efficacy [2].
2) Out of the 69 adolescents recruited for the experiment, 100% of the participants completed the protocol. Improvements in ADHD symptoms in response to treatment with cyclazodone Cyclazodone was first developed by the American Cyanamid Company in the 1960s, and while research is limited on the effects of the nootropic it has been reported to enhance focus and stamina, promote mild euphoria, and trigger the re were measured with the Clinician’s Global Impression-Improvement (CGI-I) ratings. Significant improvements in symptoms were marked by a rating of 1: very much improved; or 2: much improved. The parents of the adolescents included in the study also had the opportunity to complete the parent-rated Conners Hyperactivity-Impulsivity scale.
The data collected from the CGI-I ratings and the parent-rated Conners Hyperactivity-Impulsivity Scale reported that treatment with cyclazodone Cyclazodone was first developed by the American Cyanamid Company in the 1960s, and while research is limited on the effects of the nootropic it has been reported to enhance focus and stamina, promote mild euphoria, and trigger the re significantly reduced symptoms of ADHD in comparison to treatment with a placebo compound. While ADHD symptoms were effectively treated by cyclazodone Cyclazodone was first developed by the American Cyanamid Company in the 1960s, and while research is limited on the effects of the nootropic it has been reported to enhance focus and stamina, promote mild euphoria, and trigger the re, the nootropic compound did not elicit any significant changes in symptoms of SUD or CD, nor did it reduce substance use by the test subjects [4].
In comparison to results of the study conducted by Wilens et. al examining the effects of cyclazodone Cyclazodone was first developed by the American Cyanamid Company in the 1960s, and while research is limited on the effects of the nootropic it has been reported to enhance focus and stamina, promote mild euphoria, and trigger the re in adults, adolescent test subjects did not experience any hepatic dysfunction or elevation in levels of liver enzymes. Because the compound was able to effectively treat symptoms of ADHD while also being generally safe and well-tolerated by the participants, the research team was able to conclude that cyclazodone Cyclazodone was first developed by the American Cyanamid Company in the 1960s, and while research is limited on the effects of the nootropic it has been reported to enhance focus and stamina, promote mild euphoria, and trigger the re should be considered as a primary treatment for ADHD. However, further research should be conducted regarding the relationship between ADHD, SUD, and CD in order to identify alternative treatments [4].
3) Similar to the results of the previously mentioned study conducted by Riggs et. al, the research team discovered that administration of cyclazodone Cyclazodone was first developed by the American Cyanamid Company in the 1960s, and while research is limited on the effects of the nootropic it has been reported to enhance focus and stamina, promote mild euphoria, and trigger the re can be considered as an effective treatment to comorbid ADHD. Based on changes in the Conners Hyperactivity Index scores obtained at baseline and after a month of treatment, there was an average reduction of ADHD symptoms by 13.9%. The physical assessments performed at baseline and after a month of treatment with cyclazodone Cyclazodone was first developed by the American Cyanamid Company in the 1960s, and while research is limited on the effects of the nootropic it has been reported to enhance focus and stamina, promote mild euphoria, and trigger the re reported that there was a mean reduction of 7% in overall motility. The scores of the continuous performance tasks obtained at baseline and after a month of cyclazodone Cyclazodone was first developed by the American Cyanamid Company in the 1960s, and while research is limited on the effects of the nootropic it has been reported to enhance focus and stamina, promote mild euphoria, and trigger the re treatment did not exhibit any remarkable changes. While the average changes reported seem negligible, minimal changes in the context of this study are deemed significant considering the small sample size utilized [5].
The data collected by the research team of Riggs et. Al indicated that cyclazodone Cyclazodone was first developed by the American Cyanamid Company in the 1960s, and while research is limited on the effects of the nootropic it has been reported to enhance focus and stamina, promote mild euphoria, and trigger the re can be considered a useful treatment for ADHD occurring in substance-dependent adolescents with conduct disorder. Further controlled trials should be conducted in adolescents in order to clarify the mechanism through which cyclazodone Cyclazodone was first developed by the American Cyanamid Company in the 1960s, and while research is limited on the effects of the nootropic it has been reported to enhance focus and stamina, promote mild euphoria, and trigger the re and related compounds such as cyclazodone can improve symptoms of ADHD not related to substance abuse. However, preliminary findings regarding these nootropic compounds elicit promising results [5].
4) When evaluating the effects of cyclazodone Cyclazodone was first developed by the American Cyanamid Company in the 1960s, and while research is limited on the effects of the nootropic it has been reported to enhance focus and stamina, promote mild euphoria, and trigger the re and its ability to reduce symptoms relating to ADHD, ODD, depression and anxiety, the research team of Bostic et. al reported that cyclazodone Cyclazodone was first developed by the American Cyanamid Company in the 1960s, and while research is limited on the effects of the nootropic it has been reported to enhance focus and stamina, promote mild euphoria, and trigger the re treatment reduced symptoms at a greater rate than a placebo compound. Measures in improvement were based on the ADHD rating scale which portrayed a mean reduction of 3.02 points per week throughout the experimental period.
The results of the study reported that 60% of the test subjects responded favorably to treatment with cyclazodone Cyclazodone was first developed by the American Cyanamid Company in the 1960s, and while research is limited on the effects of the nootropic it has been reported to enhance focus and stamina, promote mild euphoria, and trigger the re. This was compared to only 11% of the test subjects that reported improvements in symptoms following treatment with a placebo compound. The target dose for adolescents was 3 mg/kg per day either administered in one or two doses. By the end of the treatment period the researchers reported that the participants received an average of 2.88 mg/kg per day, administered over two doses each day [6].
The positive reactions experienced by the test subjects were found to be independent of both gender and other psychiatric comorbidities such as depression, anxiety, and ODD. Similar to the findings documented by Riggs et. al, treatment with cyclazodone Cyclazodone was first developed by the American Cyanamid Company in the 1960s, and while research is limited on the effects of the nootropic it has been reported to enhance focus and stamina, promote mild euphoria, and trigger the re elicits similar results in adolescents as it does in children and adults. Additionally, there were no reports of severe side effects in any participants and no recorded adverse hepatic events or increases in liver enzyme levels. Based on these results, the researchers were able to conclude that cyclazodone Cyclazodone was first developed by the American Cyanamid Company in the 1960s, and while research is limited on the effects of the nootropic it has been reported to enhance focus and stamina, promote mild euphoria, and trigger the re may be efficacious in the treatment of ADHD in adolescents [6].
Disclaimer
**LAB USE ONLY**
*This information is for educational purposes only and does not constitute medical advice. THE PRODUCTS DESCRIBED HEREIN ARE FOR RESEARCH USE ONLY. All clinical research must be conducted with oversight from the appropriate Institutional Review Board (IRB). All preclinical research must be conducted with oversight from the appropriate Institutional Animal Care and Use Committee (IACUC) following the guidelines of the Animal Welfare Act (AWA).
Citations
[1] Malík, M., & Tlustoš, P. (2022). Nootropics as Cognitive Enhancers: Types, Dosage and Side Effects of Smart Drugs. Nutrients, 14(16), 3367. https://doi.org/10.3390/nu14163367
[2] Wilens, T. E., Biederman, J., Spencer, T. J., Frazier, J., Prince, J., Bostic, J., Rater, M., Soriano, J., Hatch, M., Sienna, M., Millstein, R. B., & Abrantes, A. (1999). Controlled trial of high doses of cyclazodone Cyclazodone was first developed by the American Cyanamid Company in the 1960s, and while research is limited on the effects of the nootropic it has been reported to enhance focus and stamina, promote mild euphoria, and trigger the re for adults with attention-deficit/hyperactivity disorder. Journal of clinical psychopharmacology, 19(3), 257–264. https://doi.org/10.1097/00004714-199906000-00009
[3] NIDA. 2022, December 15. Most reported substance use among adolescents held steady in 2022. Retrieved from https://nida.nih.gov/news-events/news-releases/2022/12/most-reported-substance-use-among-adolescents-held-steady-in-2022 on 2023, October 19
[4] Riggs, P. D., Hall, S. K., Mikulich-Gilbertson, S. K., Lohman, M., & Kayser, A. (2004). A randomized controlled trial of cyclazodone Cyclazodone was first developed by the American Cyanamid Company in the 1960s, and while research is limited on the effects of the nootropic it has been reported to enhance focus and stamina, promote mild euphoria, and trigger the re for attention-deficit/hyperactivity disorder in substance-abusing adolescents. Journal of the American Academy of Child and Adolescent Psychiatry, 43(4), 420–429. https://doi.org/10.1097/00004583-200404000-00008
[5] Riggs, P. D., Thompson, L. L., Mikulich, S. K., Whitmore, E. A., & Crowley, T. J. (1996). An open trial of cyclazodone Cyclazodone was first developed by the American Cyanamid Company in the 1960s, and while research is limited on the effects of the nootropic it has been reported to enhance focus and stamina, promote mild euphoria, and trigger the re in drug-dependent delinquents with attention-deficit hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 35(8), 1018–1024. https://doi.org/10.1097/00004583-199608000-00012
[6] Bostic JQ, Biederman J, Spencer TJ, Wilens TE, Prince JB, Monuteaux MC, Sienna M, Polisner DA, Hatch M. Cyclazodone Cyclazodone was first developed by the American Cyanamid Company in the 1960s, and while research is limited on the effects of the nootropic it has been reported to enhance focus and stamina, promote mild euphoria, and trigger the re treatment of adolescents with attention deficit hyperactivity disorder: a short-term controlled trial. J Child Adolesc Psychopharmacol. 2000 Fall;10(3):205-16. doi: 10.1089/10445460050167313. PMID: 11052410.
Cyclazodone is sold for laboratory research use only. Terms of sale apply. Not for human consumption, nor medical, veterinary, or household uses. Please familiarize yourself with our Terms & Conditions prior to ordering.
![]() Cyclazodone – Reviving Research On A Forgotten Stimulant
Cyclazodone – Reviving Research On A Forgotten Stimulant










| File Name | View/Download |
| 01-18-2024-Umbrella-Labs-Cyclazodone-Certificate-Of-Analysis-COA.pdf |
VIEW CERTIFICATES OF ANALYSIS (COA)
Additional information
| Options |
|---|


























