TESAMORELIN PEPTIDE 2MG VIAL
$50.99
Tesamorelin is sold for laboratory research use only. Terms of sale apply. Not for human consumption, nor medical, veterinary, or household uses. Please familiarize yourself with our Terms & Conditions prior to ordering.
- Description
- Additional information
Description
Tesamorelin Peptide
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
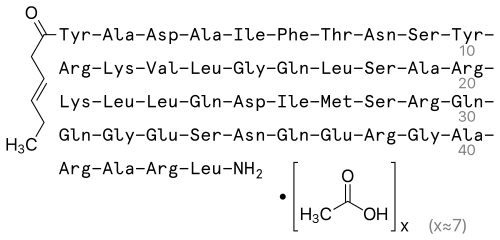
| CAS Number | 218949-48-5 |
| Other Names | Egrifta, TH9507, 804475-66-9, TH 9507 |
| IUPAC Name | (4S)-4-[[2-[[(2S)-5-amino-2-[[(2S)-5-amino-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S,3S)-2-[[(2S)-2-[[(2S)-5-amino-2-[[(2S)-2-[[(2S)-2-[[(2S)-6-amino-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-5-amino-2-[[2-[[(2S)-2-[[(2S)-2-[[(2S)-6-amino-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-4-amino-2-[[(2S,3R)-2-[[(2S)-2-[[(2S,3S)-2-[[(2S)-2-[[(2S)-3-carboxy-2-[[(2S)-2-[[(2S)-2-[[(E)-hex-3-enoyl]amino]-3-(4-hydroxyphenyl)propanoyl]amino]propanoyl]amino]propanoyl]amino]propanoyl]amino]-3-methylpentanoyl]amino]-3-phenylpropanoyl]amino]-3-hydroxybutanoyl]amino]-4-oxobutanoyl]amino]-3-hydroxypropanoyl]amino]-3-(4-hydroxyphenyl)propanoyl]amino]-5-carbamimidamidopentanoyl]amino]hexanoyl]amino]-3-methylbutanoyl]amino]-4-methylpentanoyl]amino]acetyl]amino]-5-oxopentanoyl]amino]-4-methylpentanoyl]amino]-3-hydroxypropanoyl]amino]propanoyl]amino]-5-carbamimidamidopentanoyl]amino]hexanoyl]amino]-4-methylpentanoyl]amino]-4-methylpentanoyl]amino]-5-oxopentanoyl]amino]-3-carboxypropanoyl]amino]-3-methylpentanoyl]amino]-4-methylsulfanylbutanoyl]amino]-3-hydroxypropanoyl]amino]-5-carbamimidamidopentanoyl]amino]-5-oxopentanoyl]amino]-5-oxopentanoyl]amino]acetyl]amino]-5-[[(2S)-1-[[(2S)-4-amino-1-[[(2S)-5-amino-1-[[(2S)-1-[[(2S)-1-[[2-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-amino-4-methyl-1-oxopentan-2-yl]amino]-5-carbamimidamido-1-oxopentan-2-yl]amino]-1-oxopropan-2-yl]amino]-5-carbamimidamido-1-oxopentan-2-yl]amino]-1-oxopropan-2-yl]amino]-2-oxoethyl]amino]-5-carbamimidamido-1-oxopentan-2-yl]amino]-4-carboxy-1-oxobutan-2-yl]amino]-1,5-dioxopentan-2-yl]amino]-1,4-dioxobutan-2-yl]amino]-3-hydroxy-1-oxopropan-2-yl]amino]-5-oxopentanoic acid |
| Molecular Formula | C₂₂₁H₃₆₆N₇₂O₆₇S |
| Molecular Weight | 5136 |
| Purity | ≥99% Pure (LC-MS) |
| Liquid Availability | N/A |
| Powder Availability | |
| Storage Condition | Store cold, keep refrigerated. Do NOT freeze. |
| Terms | All products are for laboratory developmental research USE ONLY. Products are not for human consumption. |
**Important Information: Each peptide comes lyophilized/freeze-dried and must be reconstituted with Bacteriostatic Water in order to be dispensable in liquid form.
What is Tesamorelin?
Tesamorelin is a peptide that is considered a Growth Hormone Releasing Hormone (GHRH) agonist, found to reduce levels of abdominal fat as well as HIV-induced lipodystrophy. When administered subcutaneously, tesamorelin is found to increase levels of glucose and lipid metabolism.
The peptide is a synthetically made polypeptide composed of 44 amino acids. The peptide differs from natural GHRH as it has been modified for optimal stability by manipulating the N terminus of the compound. Due to its similarity to GHRH, tesamorelin prompts release of Growth Hormone (GH) from the pituitary gland to act on various cells in the body. Additionally, the GH release triggers hepatocytes to secrete IGF-1 which assists in the regulation of overall growth, cell death, and lipolysis [1].
Main Research Findings
1) Tesamorelin has the potential to improve the quality of fat regardless of whether any changes in fat quantity occur.
2) Levels of visceral adipose tissue significantly decreased in ART-treated HIV patients after administration of tesamorelin
Selected Data
1) The research team of Lake et. Al examined how tesamorelin the density of adipose tissue. Participants of the study were adults aged 18-65 years old, living with HIV with evidence of central adiposity. Central adiposity was defined as a waist circumference/waist-to-hip ratio greater than 95 cm in male subjects and greater than 94 cm in female subjects. All participants has a CD4+ T lymphocyte count greater than 100 cells/mm^3, as well as HIV-1 RNA less than 10,000 copies/mL. Experimental groups were randomized and each subject received either a 2 mg dose of tesamorelin or a placebo [2].
Treatment was considered effective if there was a decrease in visceral adipose tissue greater than 8%. CT cross-sectional imaging was used to estimate both the quantity and quality of the subjects’ adipose tissue. Prior to the initiation of the study, all participants underwent single-slice, abdominal L4-L5 Ct scans in order to assess adipose tissue. This assessment was repeated after 26 weeks of following experimental procedure. The CT scans were then re-analyzed by a blinded reader that used a semi-automatic segmentation image in order to observe the density of the adipose tissue. Density of the adipose tissue was defined by CT attenuation of -190 to -30 HU. A more negative value indicates a lower density.
Considering that this experiment is a phase 3 continuation of 2 previous studies examining the effectiveness of tesamorelin against adipose tissue in individuals living with HIV, all biomarkers measured in the parent studies were also used in this trial. Measurements included total cholesterol, HDL cholesterol, triglycerides, adiponectin, C-reactive protein (CRP), activity of plasminogen activator inhibitor (PAI-1), activity of tissue plasminogen activator (TPA), and insulin-like growth factor (IGF-1). Additionally, insulin resistance was measured using the homeostatic assessment model (HOMA-IR), and was defined as fasting plasma glucose x fasting serum insulin [2].
Furthermore, serum PAI-1 antigen and tPA antigen levels were assessed by evaluation of blood samples drawn in CTAD vacutainers using ELISA assay kits obtained from Biopool, Umeea, Sweden. CTAD vacutainers contained sodium citrate, theophylline, adenosine, and dipyridamole. Testing revealed that the intra-assay coefficient of variation for the PAI-1 antigen ranged from 3.6-16.9% and the inter-assay coefficient of variation was 4.3-6.8%. For the tPA antigen assay the intra-assay coefficient of variation ranged from 7.8-9.5% while the inter-assay coefficient of variation was 4.6-9.7%. In addition to the tPA antigen and PAI-1 antigen, plasma CRP was evaluated by nephelometry which revealed a mean coefficient of variation of 6.4%, while serum adiponectin levels were measured through an ELISA assay kit. The intra-assay coefficient of variance was 3.0-5.3% and the inter-assay coefficient of variation ranged from 6.3-7.6%.
In terms of statistical analysis, changes of the density of adipose tissue from baseline to week 26 were compared via Wilcoxon matched-pairs signed-rank test. Models of linear regression were fit to compare changes in the density of adipose tissue between participants treated with tesamorelin versus those administered a placebo compound, over the 26-week experimental treatment period. Partial correlation coefficients were used to observe any association between changes in the density of adipose tissue as well as any changes in the biomarkers measured by the research team. Finally, Stata statistical software program was used to perform all statistical analyses included in this study [2].
2) The typical treatment for individuals diagnosed with HIV is antiretroviral therapy (ART). However, ART has been shown to significantly increase visceral abdominal fat while decreasing subcutaneous abdominal fat. These changes are often related to various metabolic abnormalities that can potentially increase the risk for cardiovascular disease throughout this specific population. That being said, the research team of Falutz et. Al examined the potential of the peptide, tesamorelin, to increase growth hormone secretion, reduce excess visceral abdominal fat, and improve overall metabolism. It’s important to mention that this study was modeled after two randomized phase-3 trials performed examining the efficacy of tesamorelin. Test subject entries include: HIV diagnosis, aged 18-65, CD4 cell counts higher than 100 cells/mm^3, viral load less than 10,000 copies/mL, and stable ART treatment for 8 weeks with evidence of increased abdominal fat via waist circumference measurement [3].
The subjects were randomized and subcutaneously administered either a 2 mg dose of tesamorelin, or an identical dose of a placebo compound. The investigations, sponsors, and patients were all blind to the treatment assignments. Both tesamorelin and the placebo were provided by Theratechnologies Inc. as lyophilized powder in two 3.0 mL vials. Each patient was instructed to reconstitute the powder using 1.1 mL of sterile water and self-administer the drug via subcutaneous injection of the period of the study. Over a 26 week experimental period, the primary efficacy phase included test subject assessments at weeks 6, 13, 19, and 26. All participants that completed the primary efficacy phase were asked to participate in a 26-week extension phase as well. The patients were randomized for a second time and either continued administration of tesamorelin (T-T), or switched from tesamorelin to administration of a placebo compound (T-P). Assessments of the subjects in the extension phase took place at weeks 32, 39, 45, and 52 [3].
Compliance to the treatment was assessed by reporting the number of used, unused, or broken vials each patient returned at the study visit. Safety of the patients was maintained by discontinuing subjects that were asymptomatic with a fasting glucose over 180 mg/dL or symptomatic with a fasting glucose lower than 180 mg/dL, in order to account for hyper- or hypersensitivity reactions. All body composition, biochemical testing, and body image parameters were determined by predetermined standards. Furthermore, all statistical analyses were performed by Octagon Research Solutions. After consulting the U.S. Food and Drug Administration, it was determined that 8% was the minimum difference needed to detect a clinically significant difference in visceral abdominal fat between patients treated with tesamorelin versus the placebo. Secondary endpoints measured by the research team included: IGF-1 levels, triglycerides, the ratio of total cholesterol to high-density lipoprotein cholesterol, and any patients-reported outcomes related to body composition.
After gathering all available data on each patient included in the trial, the baseline values of each variable was compared using an ANOVA model including the study and treatment group. The primary efficacy endpoint was measured using an ANCOVA model in order to observe the natural log ratio of visceral abdominal fat at 26 weeks in comparison to baseline levels. A highly stringent analysis was used in order to account for type 1 errors, meaning that a resulting p value of less than 0.001 would be deemed statistically significant. Additional sensitivity analyses were performed through the use of the mixed-model repeated-measure method, followed by an ANCOVA model to analyze the change in lipid levels from baseline to week 26. The ANCOVA model also took into account the use of any lipid-lowering treatments, belly size evaluation change score, belly appearance distress, and belly profile [3].
After the initial 26 week treatment period, eligible subjects moved on to the safety extension phase. Comparisons of the level of visceral abdominal fat and all secondary variables were performed through the use of repeated-measures ANOVA. The clinical laboratory values for both stages of the study were categorized by treatment group and summarized using descriptive statistics. The research team also recorded the frequency of adverse events and serious adverse events in both the primary study and the extended study [3].
Discussion
1) The main purpose of this study was to see how tesamorelin affects the quality and quantity of adipose tissue in individuals living with HIV. Results of the study reported that tesamorelin significantly reduced visceral adipose tissue, in comparison to the individuals treated with a placebo. The effects of tesamorelin were maintained after the research team adjusted the dosage of the peptide for baseline density of adipose tissue, baseline area of adipose tissue, and changes in the area of adipose tissue. Administration of tesamorelin was shown to independently improve visceral and subcutaneous adipose tissue functioning. The researchers hypothesized that increased density of both visceral and subcutaneous adipose tissue is associated with decreased in the area of visceral and subcutaneous adipose tissue, increases in circulating adiponectin levels, and improved levels of total cholesterol and triglycerides [2].
Adiponectin is typically secreted by adipose tissue and is crucial to regulation of insulin sensitivity. The accumulation of visceral adipose tissue tends to suppress adiponectin levels, resulting in insulin resistance, vascular dysfunction, and systemic inflammation. When measuring changes in adiponectin levels, the researchers found that over the 26 week experimental period greater increases in adiponectin were related to greater increases in fat quality. The reported results of the study also displayed benefits on the quality of both visceral and subcutaneous fat in a manner independent of any changes in the quantity of adipose tissue. These findings were also associated with improvements in circulating levels of adiponectin, total cholesterol, and concentration of triglycerides.
Based on the effects elicited on the quality of visceral and subcutaneous adipose tissue, the research team suggested that tesamorelin has the potential to play a unique role in improving the functioning of adipose tissue, as well as regulating cardiometabolic risk in the test subjects diagnosed with HIV. Considering that there was no significant correlation between the density of adipose tissue and the insulin resistance analysis that was performed, further research should be conducted on this topic considering the multi-factoral qualities of insulin resistance in individuals with HIV [2].
2) The primary efficacy phase of the study conducted by the research team of Falutz et. Al, resulted in 543 patients administered tesamorelin and 263 patients administered the placebo. Out of these test subjects, 207 (79%) in the placebo group and 413 (76%) in the experimental group completed the 26 week treatment period and progressed to the extension phase.
Results of the primary efficacy phase reported a significant reduction in the amount of visceral abdominal fat in patients treated with tesamorelin, in comparison to the patients administered a placebo compound. However, while visceral adipose tissue decreased, there were no significant changes in the amount of subcutaneous adipose tissue. Similar results were reported using the mixed-model repeated-measure approach. Additionally, in the subjects treated with tesamorelin there was a noticeable decrease in trunk and limb fat, as well as waist circumference. Lean body mass was also found to have significantly increased in the tesamorelin-treated patients in comparison to placebo-treated patients. No significant changes were measured in body mass index between the experimental and placebo groups [3].
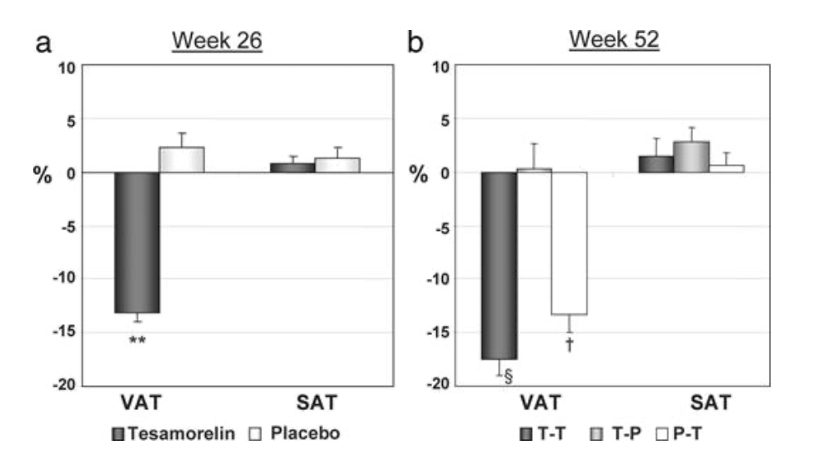
Figure 1: Changes from baseline in visceral adipose tissue and subcutaneous adipose tissue after (a) 26 weeks, and (b) 52 weeks.
The researchers found that the effects of tesamorelin on visceral adipose tissue was similar among the subjects following a baseline ART regimen with no evidence of treatment-by-ART interaction. The results of the study also briefly mentioned that treatment with tesamorelin did not lead to any statistically significant discrepancies between the reduction of visceral adipose tissue in males versus females, measuring at -13.2% and -12.5%, respectively. In the pooled analysis of the study, results reported a significant reduction in triglyceride levels and the ratio of total cholesterol to HDL cholesterol. There was a less significant but still noticeable reduction in total cholesterol, HDL cholesterol, and non-HDL cholesterol. In terms of the effects of tesamorelin on fasting glucose, fasting insulin, and 2 hour glucose on oral glucose tolerance, there were no noticeable differences detected in comparison to the placebo group [3].
ANCOVA analysis was performed and revealed similar results to the previously mentioned findings when controlled for IFG/diabetes conditions at the time of screening. That being said, there was no interaction between the treatment and IFG/diabetes condition. The patients treated with tesamorelin also exhibited a 0.14% increase from baseline in mean glycated hemoglobin levels, in comparison to the 0.02% increase noted in the placebo group. This led to an increase from 5.26% to 5.39% in the tesamorelin-treated patients. Patients treated with tesamorelin also experienced a significant increase in mean IGF-1 levels within the physiological range.
Furthermore, administration of tesamorelin led to an overall improvement in body image throughout the subjections. Changes in body image were determined by physician-rated belly profile, belly appearance distress, patient-reported belly profile, and the global analog scale representing health-related quality of life (HRQOL). Patients treated with a placebo exhibited deterioration of HRQOL, however, the patients treated with tesamorelin experienced no deviations from baseline. The researchers concluded that improvements in body image were related to the degree of reduction in visceral adipose tissue in the subjects treated with tesamorelin [3].
Results from the extension phase of the study reported improvements in the levels of visceral adipose tissue, trunk fat, waist circumference, triglycerides, total cholesterol, and IGF-1, at week 52 in the T-T group, while subcutaneous adipose tissue and limb fat were maintained. No significant changes were observed in glucose or insulin levels at week 52 in the T-T group. In the T-P group, the subjects regained visceral abdominal fat after switching from the peptide to the placebo. By week 52 there was no significant difference from baseline levels. Similar changes were noticed when measuring trunk fat, however, waist circumference did not significantly change. Patient and physician-rated belly appearance and belly profile continued to improve in the T-T groups, however, these improvements regressed in T-P groups [3].
Disclaimer
**LAB USE ONLY**
*This information is for educational purposes only and does not constitute medical advice. THE PRODUCTS DESCRIBED HEREIN ARE FOR RESEARCH USE ONLY. All clinical research must be conducted with oversight from the appropriate Institutional Review Board (IRB). All preclinical research must be conducted with oversight from the appropriate Institutional Animal Care and Use Committee (IACUC) following the guidelines of the Animal Welfare Act (AWA).
Citations
[1] LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-. Tesamorelin. [Updated 2018 Oct 20]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK548730/
[2] Lake JE, La K, Erlandson KM, Adrian S, Yenokyan G, Scherzinger A, Dubé MP, Stanley T, Grinspoon S, Falutz J, Mamputu JC, Marsolais C, McComsey GA, Brown TT. Tesamorelin improves fat quality independent of changes in fat quantity. AIDS. 2021 Jul 15;35(9):1395-1402. doi: 10.1097/QAD.0000000000002897. PMID: 33756511; PMCID: PMC8243807.
[3] Falutz J, Mamputu JC, Potvin D, Moyle G, Soulban G, Loughrey H, Marsolais C, Turner R, Grinspoon S. Effects of tesamorelin (TH9507), a growth hormone-releasing factor analog, in human immunodeficiency virus-infected patients with excess abdominal fat: a pooled analysis of two multicenter, double-blind placebo-controlled phase 3 trials with safety extension data. J Clin Endocrinol Metab. 2010 Sep;95(9):4291-304. doi: 10.1210/jc.2010-0490. Epub 2010 Jun 16. PMID: 20554713.
Peptides Prefer the Cold
In order to reduce peptide breakdown, keep peptides refrigerated at all times but DO NOT FREEZE.
Swab the top of the vial with a 95% alcohol wipe before accessing.
Only Mix with Sterile Bacteriostatic Water
Bacteriostatic water is vital to preventing contamination and preserving the stability of the compound.
Push the needle through the stopper at an angle to direct the stream to the side of the vial.
Reconstituted peptide solution should be stored around 4 degrees Celsius but not frozen, while lyophilized peptide solution should be kept at -20 degrees Celsius.
Tesamorelin is sold for laboratory research use only. Terms of sale apply. Not for human consumption, nor medical, veterinary, or household uses. Please familiarize yourself with our Terms & Conditions prior to ordering.



| File Name | View/Download |
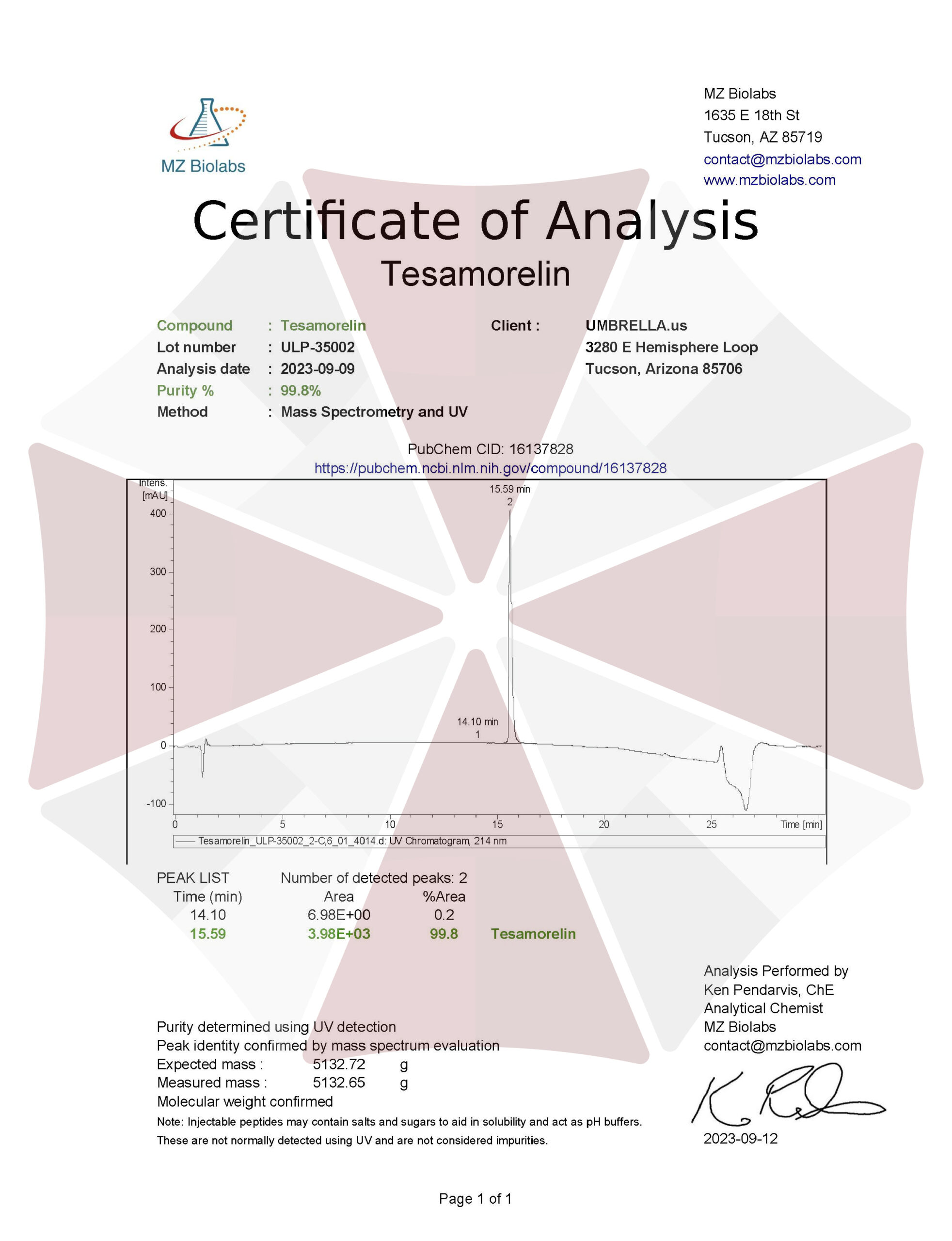
| 09-09-2023-Umbrella-Labs-Tesamorelin-Certificate-Of-Analysis-COA.pdf |
VIEW CERTIFICATES OF ANALYSIS (COA)
Additional information
| Weight | 1 oz |
|---|---|
| Dimensions | 0.5 × 0.5 × 1 in |