SARMS for Sale: RAD 140 TESTOLONE SARM POWDER – 1000MG / 1 GRAM
$70.99
RAD-140 SARM is sold for laboratory research use only. Terms of sale apply. Not for human consumption, nor medical, veterinary, or household uses. Please familiarize yourself with our Terms & Conditions prior to ordering.
*Includes:
- One (~10mg – 15mg) Red Micro Scoop
Also Available In:
![]() Liquid Option >>
Liquid Option >>
![]() Gel Option >>
Gel Option >>
- Description
- Additional information
Description
RAD 140 TESTOLONE SARM POWDER FOR SALE
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
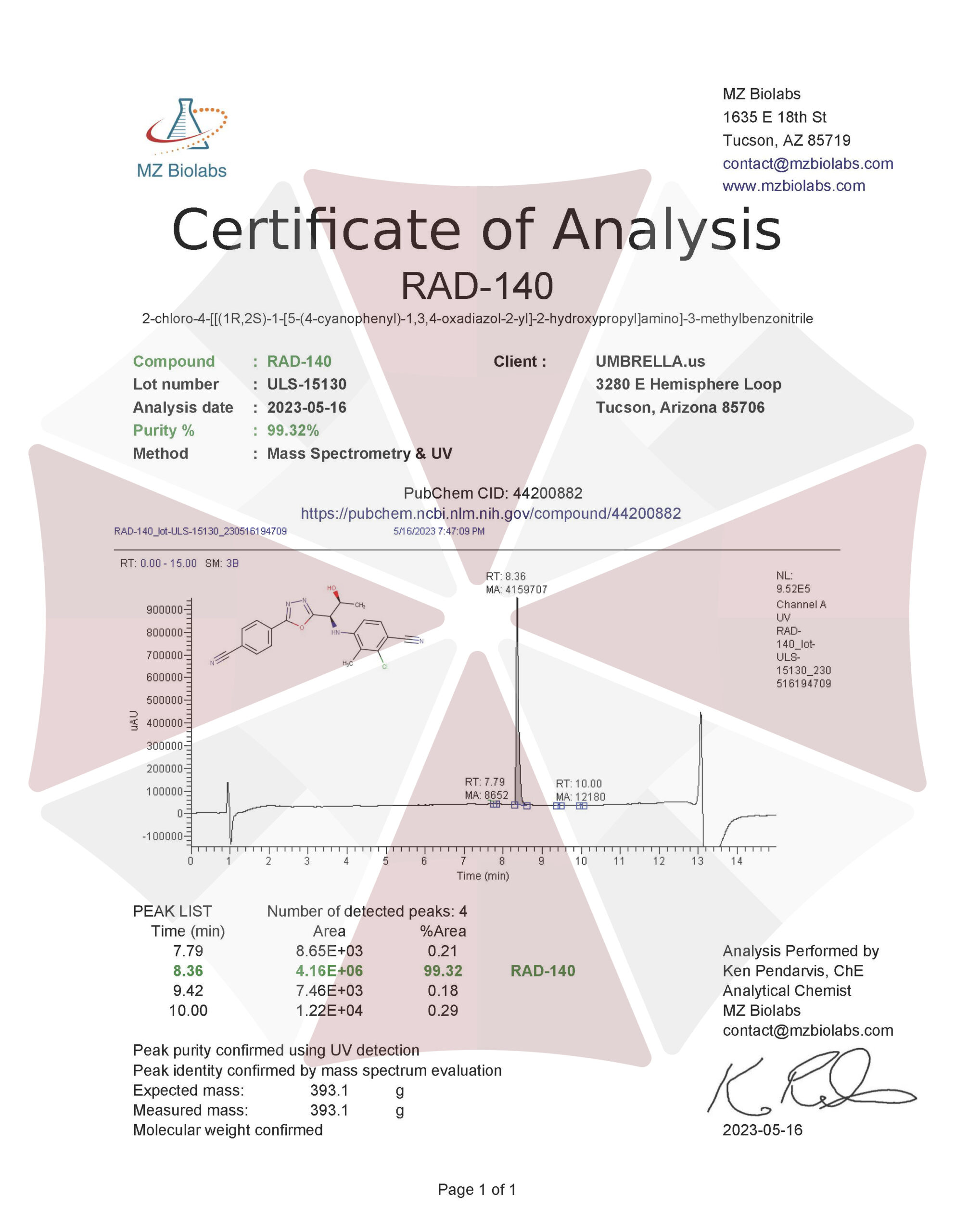
| CAS Number | 1182367-47-0 |
| Other Names | Testolone, RAD140, RAD 140, Vosilasarm, 4O87Q44KNC, CHEMBL1672635, D09ZLZ |
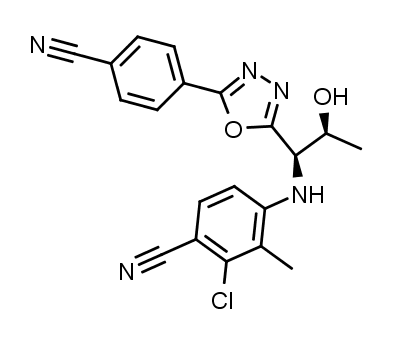
| IUPAC Name | 2-chloro-4-[[(1R,2S)-1-[5-(4-cyanophenyl)-1,3,4-oxadiazol-2-yl]-2-hydroxypropyl]amino]-3-methylbenzonitrile |
| Molecular Formula | C₂₀H₁₆ClN₅O₂ |
| Molecular Weight | 393.8 |
| Purity | ≥99% Pure (LC-MS) |
| Liquid Availability |
|
| Powder Availability | |
| Gel Availability | |
| Storage | Store in cool dry environment, away from direct sunlight. |
| Terms | All products are for laboratory developmental research USE ONLY. Products are not for human consumption. |
*Includes:
- One (~10mg – 15mg) Red Micro Scoop
What is RAD 140?
(2-chloro-4-[[(1R,2S)-1-[5-(4-cyanophenyl)-1,3,4-oxadiazol-2-yl]-2-hydroxypropyl]amino]-3-methylbenzonitrile, also referred to as testolone or RAD140, is a selective androgen receptor modulator (SARM). Due to its ability to bind to androgen receptors, the SARM has been shown to mimic the action of testosterone. SARMs are becoming more popular and widely available for research purposes as evidence shows the compounds do not elicit negative androgenic side effects, like those observed with testosterone treatment.
RAD140 was originally developed by a research team made of individuals from the University of Illinois, Obiter Research, and Cambridge Major Laboratories. This project was completed in conjunction with Radius Health Incorporated. Current studies regarding the use of RAD140 have concluded that, like testosterone, the SARM promotes anabolism of bone and muscle tissue. The positive reported results have urged researchers to investigate the role of RAD140 in stimulating antitumor activity and enhancing neuroprotection [1].
Main Research Findings
1) Treatment with RAD140 leads to enhanced neuroprotection in hippocampal cell cultures as well as improved anabolic activity in the levator ani muscle.
2) RAD140 treatment led to an increased stimulation of the levator ani muscle in a manner similar to testosterone treatment without significant stimulation of the prostate and seminal vesicles.
3) Researchers LuRusso et. Al determined treatment with RAD 140 was well-tolerated when administered to women with metastatic breast cancer.
Selected Data
1) The research team of Jayaraman et. Al examined the neuroprotective effects elicited by RAD 140 in response to normal age-related degeneration and dysfunction. Pregnant Sprague-Dawley rats were euthanized in order for researchers to collect the 17-18 day old pups in order to prepare neuronal cultures.The primary rat hippocampal cultures were prepared accordingly while the dissociated hippocampal neurons were plated in order to complete cell-viability assays. The cultures were prepared using a mix of approximately 12-14 both male and female in order to control for any differences based on sex. Experiments began 1-2 days in vitro and all experiments were completed at least three times with different cultures [2].
Data for this portion of the study was measured through cell-viability assays and Western blot analysis. Cell viability was assessed after the experimental treatment period was finished. Staining with vital dye calcein acetoxymethyl ester highlighted the number of viable hippocampal neurons present. The stained cells of each culture were counted in according to a predetermined, regular pattern separating the sample into 4 separate fields per well. Additionally, there were 3 wells per condition in each experiment that occurred; the amount of viable neurons in the treatment groups were compared to vehicle-treated control groups.
Using a reducing sample buffer, lysates were collected from the cultures in order for the researchers to perform a Western blot analysis. The lysates were boiled for 5 minutes and centrifuged for 10 minutes; all supernatants were analyzed by immunoblotting. Furthermore, the band densities were recorded through the use of Image J software, while the relative percent intensity of phospho-ERK bands were properly plotted on a graph [2].
The second portion of this study utilized 3 month-old male Sprague-Dawley rats that had previously been gonadectomized (GDX) or subjected to a sham-operation 14 days prior to treatment. The animals were housed in their own cage, stuck to a strict 12 hours dark, 12 hours light schedule, and given access to food and water ad libitum. The GDX animals receiving testosterone treatment received an implantation of a SILASTIC capsule packed with dry testosterone and capped with silicone glue. The same procedures were followed for the animals receiving a vehicle treatment with nothing packed into the capsule [2].
The GDX animals receiving SARM treatment were administered 1 mg/kg of RAD 140 suspended in 1 mg/mL of 0.5% methyl cellulose, via oral gavage. The treatment was administered to the different groups daily, over an experimental period of 2 weeks. On day 13 of treatment the test subjects were administered either 10 mg/kg of kainate or a sterile water control. On day 14, the final day of treatment, the SARM-treated rats were subcutaneously injected with a 1 mg/mL dose of RAD 140 suspended in safflower oil. This was due to the kainate lesion that made administration through oral gavage difficult. The animals were euthanized at the end of the treatment period; the researchers removed and hemisected the brains and prepared each half accordingly. The prostate, seminal vesicles, and levator ani muscle were then removed and dissected in order for the researchers to measure weight changes.
Results of this study were further measured through seizure assessment and immunohistochemistry tests. Behavior of the subjects was monitored by the researchers for 3 hours after administration of kainate in order to take note of aspects regarding the latency and severity of the seizures. The latency period occurs shortly after the kainate injection and is typically described as a “wet dog shake” and defined as the first appearance of stereotypic seizure-related behavior. The seizure severity of the animals was measured on a scale from 0-5, ranging from no seizure activity to seizure accompanied by rearing and falling [2].
The immunohistochemistry test examined the number of immunoreactive cells present in the fixed brain samples. Every eighth brain section was immunostained with NeuN antibody, mounted on slides, and allowed to dry overnight prior to further analysis. 2-dimensional cell counts of random sampling were used to estimate the number of immunoreactive NeuN cells present in the CA2/3 region of the hippocampus. Every eighth hippocampal section had the CA2/CA3 region of the hippocampus outlined in order to organize an oriented counting frame. Only positively stained nuclei were recorded, and to control for variability throughout the analyzed sections, the number of immunoreactive nuclei counted was divided by the number of sections. The resulting figure was defined as the control variable and expressed as the percentage of neurons counted in the sham-GDX, non-lesioned group. All statistical analyses that occurred to compare the treatment groups were performed using ANOVA and Fisher’s least significant difference test [2].
2) Initially, RAD 140 was characterized through the use of in vivo assay in order to determine its efficacy on androgenic activity in both castrated and intact male rats. Young castrated male rats were preferred in this experiment because they still qualified as androgen-naive. Essentially, the rats provided a blank canvas for the researchers to examine the effects elicited by RAD 140 and testosterone on androgenic activity.
Based on the results of the initial experiment the research team of BLANK et. Al observed any potential antagonist effects RAD 140 elicits on testosterone propionate (TP). Additionally they examined the changes found in the levator ani muscle when the castrated rats were administered a combination treatment of RAD 140 and TP [3].
In order to gain a full understanding of the androgenic effects of RAD 140 compared to testosterone, the researchers turned their attention to young intact male rats that have only slightly reduced levels of endogenous testosterone. This results in the retention of prostate sensitivity, however, baseline stimulation is similar to levels observed in the castrated rats. The intact rats were given doses of TP and RAD 140 varying from 0.1 mg/kg to 30 mg/kg in order to record the response in muscle growth [3].
3) The first phase 1 clinical study regarding the efficacy of RAD140 was conducted by the research team of LoRusso et. Al. The purpose of their experiment was to determine the safety and tolerability of the SARM, as well as to examine the antitumor effects of RAD140 in cases of postmenopausal women with advanced breast cancer. Researchers measured the occurrences of dose-limiting toxicity in order to estimate a recommended dose for expansion (RDE) and maximum tolerated dose (MTD). The secondary goal of the study was to develop a pharmacokinetic profile of the compound. RAD140 was administered in a 3 + 3 dose-escalation phase to subjects diagnosed with inoperable metastatic ER+/HER2- breast cancer. Administration of the SARM was followed by a pharmacokinetic expansion (PKE) [4].
There were approximately 30 patients involved in the first treatment round of RAD 140. The compound was administered to the subjects daily in 50 mg doses in order to proceed with dose-limiting toxicity (DLT) evaluations to determine the MTD and RDE. If patients passed through the first DLT evaluation period without experiencing DLT, they continued on to the second evaluation of the study. When 2 DLTs occurred in 3-6 patients the researchers halted dosage increases. At that point, the MTD was measured as the highest treatment dose where only 1 in every 6 patients experienced only 1 DLT occurrence.
In order to determine the maximum treatment dosage, researchers added a cohort patient group administered a single-dose PK in order to gather information about the safety and tolerability of the compound. Subjects were administered one dose of the hypothesized MTD, followed by 7 days of no treatment. Plasma samples were collected from the subjects during the no treatment period so the researchers could complete PK characterization. After this sample treatment week the cohort group began daily RAD140 treatment at the preliminary MTD [4].
The safety and tolerability of RAD 140 was examined through physical examinations, vital signs, blood tests, ECG, and serum chemistry evaluations. Researchers paid close attention to any changes in serum cardiac troponin levels as an indicator of DLT. The severity of elicited side effects were graded according to the Common Terminology Criteria for Adverse Events. Furthermore, a tumor assessment was performed at baseline and every 8 weeks after, during Cycles 2-12. After Cycle 12 the tumor assessments took place every 3 months.
Additionally, the blood samples were collected to complete the PK characterization and were gathered from the subjects pre- and post-administration of RAD 140. Samples were initially collected Cycle 1 Day 1 at hours 1, 2, 4, 6 ,8, and 24, and were collected again Cycle 1 Day 15 hours 1, 2, 4, 6, and 8. Blood samples pre-dose administration were collected on Day 28 of Cycles 2-6. Pre-dose samples of the PK cohort group were collected at hours 1, 2, 4, 6, 8, 24, 48, 72, 96, and 144 hours after receiving the single-dose of PK [4].
Discussions
1) Initial results of the study found that the use of RAD140 was able to protect against neuronal death induced by A-beta-1-42. The cell cultures were exposed to A-beta for 24 hours. This resulted in a 50% reduction in viable neurons, however, when the subjects were treated with 10 nM of testosterone or DHT one hour before exposure, levels of cell death were significantly reduced. When treating the cells with 30 nM RAD 140 one hour before exposure, similar reduced levels of cell death were observed [2].
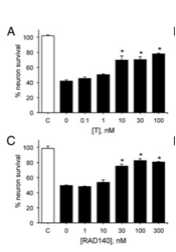
Figure 1: Changes in neuron viability in A) testosterone versus C) RAD 140
The research team also determined that androgen-mediated neuroprotection against apoptosis depends on the activation of the MAPK/ERK signaling pathway. The first step of this study determined that RAD 140 is involved in MAPK/ERK signaling by exposing the neuronal cultures to 100 nM of RAD 140 in the presence or absence of MEK inhibitor pretreatment. The next step was to observe the inhibitory effects of U0126 MAPK/ERK signaling when the cell cultures were treated with RAD 140 and protected against cell death induced by A-beta. The researchers came to the conclusion that pretreatment with U0125 blocks the neuroprotective effects of RAD 140.
Observations of the neuronal cell cultures led the research team to examine the neuroprotective effects of RAD 140 in vivo. As initial procedures of the study explained, the male rats underwent GDX or a sham-surgery and were then treated with kainate 13 days after androgen treatments began. Results reported that GDX led to a dramatic reduction in weight of all 3 androgen-responsive tissues: the prostate, seminal vesicles, and the levator ani muscle [2].
When the GDX subjects were treated with testosterone, the weight of each three tissues were restored to levels similar to those in the sham-surgery group. Treatment with RAD 140 led to a slight elevation in the weights of the prostate and the seminal vesicles. The weight of the tissues in these treatment groups were still far lower than the weight recorded in the sham surgery and GDX + testosterone groups. Furthermore, treatment with RAD 140 led to a significant increase in the weight of the levator ani muscle. The recorded measurements were similar to those identified in the sham-surgery and GDX + testosterone groups [2].
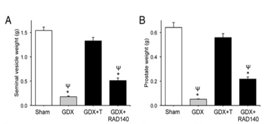
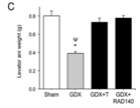
Figure 2: Changes in the weight of A) the seminal vesicles, B) prostate, and C) levator ani, in response to the different treatments
The neuroprotective effects of RAD 140 against kainate-induced neuron death were further investigated by assessing the amount of surviving immunostained cells found in the CA2/3 region of the hippocampus. In comparison to the vehicle-treated sham-operated animals, kainate alone inducted 20% cell loss. These effects were blocked when the androgen treatment was administered. The researchers reported that both testosterone and RAD 140 were effective at protecting the GDX rats against neuronal cell death induced by kainate injections.
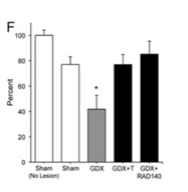
Figure 3: Percentage of cell survival in response to different treatment variables.
Additionally, the researchers examined how hormonal manipulations affect latency and severity in kainate-induced seizures. Researchers chose to measure this as the intensity of seizure behaviors are typically linked to the degree of hippocampal neuron loss experienced. Over the three hour observation period all of the animals treated with kainate had at least one seizure. However, the vehicle-treated animals did not exhibit any seizure-related behavior. Seizure latency did not show any significant changes amongst the lesioned animals; seizure severity did not exhibit any significant variations between treatment groups. However, there was a slight reduction in severity noted in the GDX + testosterone and the GDX + RAD 140 treatment groups [2].
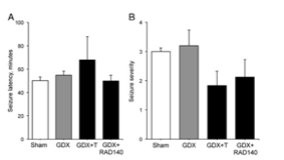
Figure 4: Changes observed in seizure latency and severity between treatment groups
2) In vivo assays found that oral administration of increasing doses of RAD 140 led to significant stimulation of the levator ani muscle. These results are seen with doses as small as 0.03 mg/kg. This is compared to the vehicle-treated control group, and the group receiving TP treatment. While it takes 3 mg/kg of RAD 140 to reach the same level of muscle growth in the levator ani as 1 mg/kg of TP, prostate growth and accompanying side effects were significantly reduced in the rats administered the SARM. The researcher thought it was important to note that RAD 140 did not produce a high level of stimulation in the prostate or seminal vesicles, regardless of how high the dose was [3].
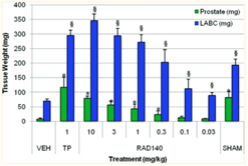
Figure 5: Prostate weight versus weight of the levator ani muscle in response to different treatments.
When examining the antagonist effects of RAD 140 against testosterone propionate in the the researchers found that doses greater than 10 mg/kg of RAD 140 antagonizes the effects elicited by 1 mg/kg of TP. However, it is important to note that the antagonistic effects of RAD 140 were seen only in the seminal vesicles; co-administering RAD 140 with TP actually led to an increase in the effects of TP, specifically in the levator ani muscle. The research team of BLANK et. Al were able to conclude that in doses of 0.3-1 mg/kg, RAD 140 elicits antagonistic effects for 1 mg/kg of testosterone. A slight reduction in stimulation due to TP was seen in the prostate when co-administered with RAD 140, however, the changes were deemed not of statistical significance. Overall, RAD 140 acts as an agonist to the levator ani and a partial antagonist to the seminal vesicles, and to a lesser extent, the prostate [3].
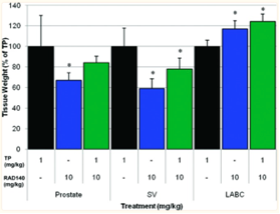
Figure 6: Visual representation of the tissue-selective activity of RAD 140
Finally, results of RAD 140 treatment in intact male rats found that the SARM increased the weight of the levator ani with no stimulation of the prostate. Weight of the levator ani muscle was shown to increase with the smallest dose of RAD 140 administered, 0.1 mg/kg. The lack of prostate stimulation was observed for every dose of RAD 140 ranging from 0.1 mg/kg to 30 mg/kg. A notable detail of the study explains that a 30 mg/kg dose of RAD 140 was needed in order to reach the same levels of prostate growth elicited with a 0.5 mg/kg dose of TP. When the subjects were given doses 0.3 mg/kg, the researchers reported that RAD 140 resulted in muscle efficacy at levels similar to that of TP administered in doses of 0.5 mg/kg [3].
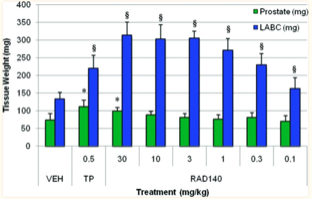
Figure 7: Weight of the levator ani in response to varying doses of TP and RAD 140
3) Results of this study first reported the safety of RAD 140 in the postmenopausal subjects. The most frequent side effects observed were increased levels of aspartate aminotransferase, alanine aminotransferases, total bilirubin, dehydration, vomiting, and weight loss. 77.3% of all patients reported having some grade of side effect. Only 31.8% of those were grade 3 and there were no grade 4 side effects identified. 41% of the patients experienced serious side effects, however, researchers were able to later determine that the instances were not likely related to the drug study. No treatment deaths were reported [4].
DLTs were reported in 6 out of 17 DLT-evaluable patients, all occurrences were measured at grade 3 but were capable of reversal. Based on the frequency of DLTs, the researchers concluded that the MTD and RDE are at approximately 100 mg, daily. Hypophosphatemia was observed in 5 out of 22 patients, however, only two of those cases were considered Grade 3, and only one was determined to be related to the drug study. All cases were reversed when treatment was momentarily paused, and when resumed at a later date there was no recurrence of the DLT [4].
The physical examinations performed by the researchers did not exhibit any significant changes. 11-DOC was defined as an indicator of cardiac inflammation and changes in the hormone biosynthetic pathways. Mean and median levels of 11-DOC did not experience any change in response to any treatment. Cardiac troponin and troponin T were both recorded in order to assess the grades of severity racing from baseline to worst post-baseline value. All patients exhibited Grade 0 troponin T levels at baseline, treatment only caused a slight elevation to Grade 1 troponin T levels.
Any adverse cardiac side effects elicited were only observed in 18.2% of patients and included Grade 2 atrial flutter and fibrillation, Grade 1 palpitations, and Grade 3 tachycardia and right ventricular failure. The patients with Grade 3 tachycardia and effusion were dismissed from the study, however, all cardiac-related side effects, apart from palpitations, were not related to RAD 140 treatment. Additionally, the research team noted that there were no significant androgenic side effects related to treatment with the SARM. Any reported side effects were limited to Grade 1 abnormal hair growth, weight gain, or acne [4].
The pharmacokinetic report determined that the original treatment group experienced an increase in plasma concentration up to 24 hours after administration of RAD 140. The additional, single-dose PK group that was created exhibited a rapid increase in RAD 140 serum levels, however, this was followed by a steady decline that formed secondary and tertiary peaks. Overall, the researchers were able to conclude that RAD 140 has an acceptable safety profile and elicits high levels of antitumor activity in pretreated postmenopausal women with advanced breast cancer. This included women who had previously been administered a CDK4/6 inhibitor or whose tumors are a result of ESR1 mutations [4].
Disclaimer
**LAB USE ONLY**
*This information is for educational purposes only and does not constitute medical advice. THE PRODUCTS DESCRIBED HEREIN ARE FOR RESEARCH USE ONLY. All clinical research must be conducted with oversight from the appropriate Institutional Review Board (IRB). All preclinical research must be conducted with oversight from the appropriate Institutional Animal Care and Use Committee (IACUC) following the guidelines of the Animal Welfare Act (AWA).
Citations
[1] Leung K, Yaramada P, Goyal P, Cai CX, Thung I, Hammami MB. RAD-140 Drug-Induced Liver Injury. Ochsner J. 2022 Winter;22(4):361-365. doi: 10.31486/toj.22.0005. PMID: 36561105; PMCID: PMC9753945.
[2] Anusha Jayaraman, Amy Christensen, V. Alexandra Moser, Rebekah S. Vest, Chris P. Miller, Gary Hattersley, Christian J. Pike, Selective Androgen Receptor Modulator RAD140 Is Neuroprotective in Cultured Neurons and Kainate-Lesioned Male Rats, Endocrinology, Volume 155, Issue 4, 1 April 2014, Pages 1398–1406, https://doi.org/10.1210/en.2013-1725
[3] Miller CP, Shomali M, Lyttle CR, O’Dea LS, Herendeen H, Gallacher K, Paquin D, Compton DR, Sahoo B, Kerrigan SA, Burge MS, Nickels M, Green JL, Katzenellenbogen JA, Tchesnokov A, Hattersley G. Design, Synthesis, and Preclinical Characterization of the Selective Androgen Receptor Modulator (SARM) RAD140. ACS Med Chem Lett. 2010 Dec 2;2(2):124-9. doi: 10.1021/ml1002508. PMID: 24900290; PMCID: PMC4018048.
[4] LoRusso P, Hamilton E, Ma C, Vidula N, Bagley RG, Troy S, Annett M, Yu Z, Conlan MG, Weise A. A First-in-Human Phase 1 Study of a Novel Selective Androgen Receptor Modulator (SARM), RAD140, in ER+/HER2- Metastatic Breast Cancer. Clin Breast Cancer. 2022 Jan;22(1):67-77. doi: 10.1016/j.clbc.2021.08.003. Epub 2021 Aug 20. PMID: 34565686.
RAD-140 SARM is sold for laboratory research use only. Terms of sale apply. Not for human consumption, nor medical, veterinary, or household uses. Please familiarize yourself with our Terms & Conditions prior to ordering.
![]() RAD-140 (Vosilasarm) – Emerging Research And Clinical Insights
RAD-140 (Vosilasarm) – Emerging Research And Clinical Insights




| File Name | View/Download |
| 05-16-2023-Umbrella-Labs-RAD-140-Testolone-Certificate-Of-Analysis-COA.pdf |