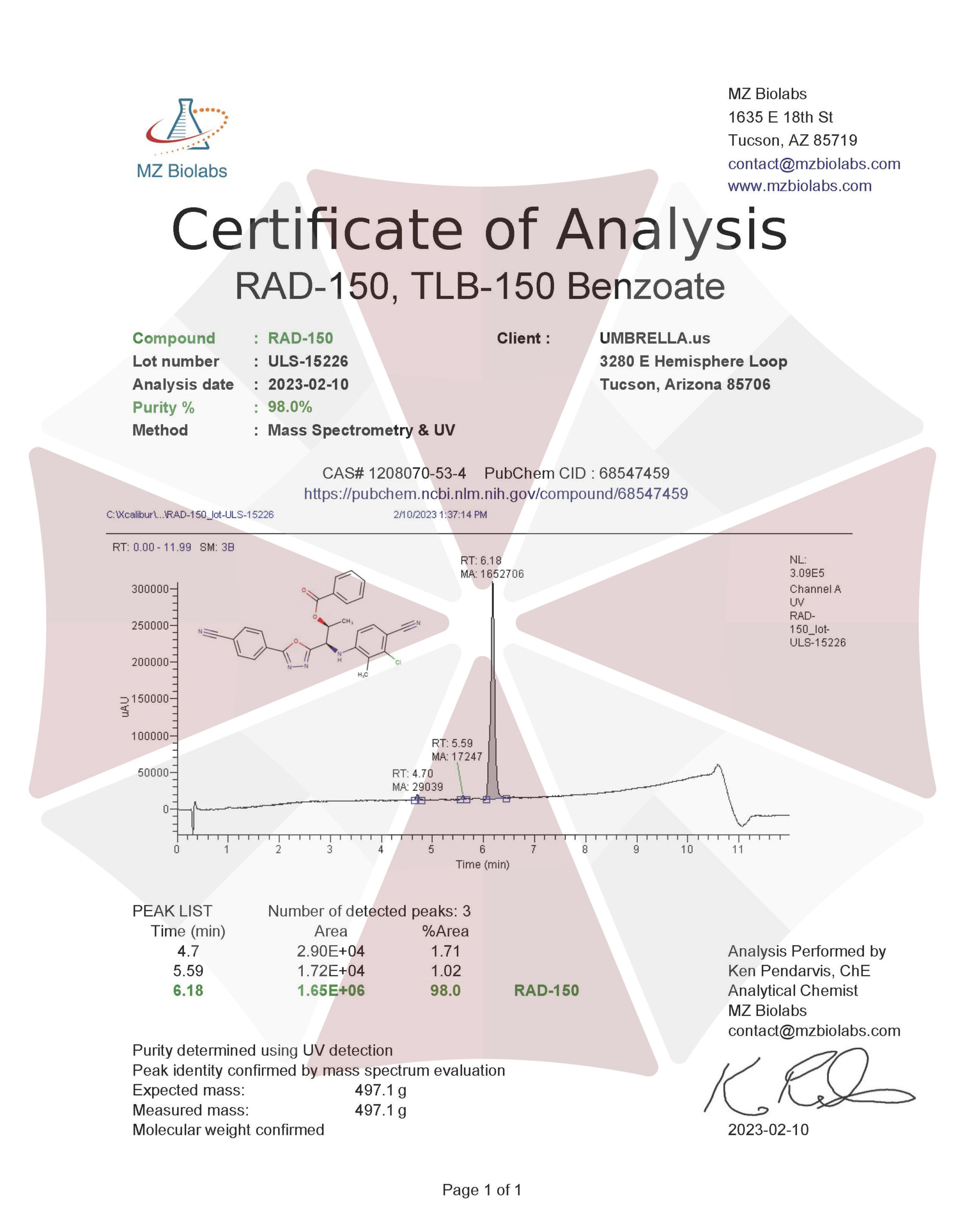
RAD 150 (TLB 150) SARM POWDER – 1000MG / 1 GRAM
$88.99
RAD-150 SARM is sold for laboratory research use only. Terms of sale apply. Not for human consumption, nor medical, veterinary, or household uses. Please familiarize yourself with our Terms & Conditions prior to ordering.
*Includes:
- One (~10mg – 15mg) Red Micro Scoop
Also Available In:
![]() Liquid Option >>
Liquid Option >>
![]() Gel Option >>
Gel Option >>
- Description
- Additional information
Description
RAD-150 SARM (aka TLB-150 or RAD-140 Ester) Powder
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
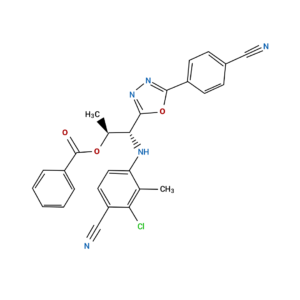
| CAS Number | 29622-29-5 |
| Other Names | RAD150, RAD 150, TLB150, TLB-150, TLB 150, DTXSID40183806, |
| IUPAC Name | 2-[5-chloropentyl(methyl)amino]-N-(2,6-dimethylphenyl)acetamide |
| Molecular Formula | C₁₆H₂₅ClN₂O |
| Molecular Weight | 296.83 |
| Purity | ≥99% Pure (LC-MS) |
| Liquid Availability | |
| Powder Availability | |
| Gel Availability | |
| Storage | Store in cool dry environment, away from direct sunlight. |
| Terms | All products are for laboratory developmental research USE ONLY. Products are not for human consumption. |
*Includes:
- One (~10mg – 15mg) Red Micro Scoop
What is RAD 150?
RAD 150, also referred to as TLB 150, is a selective androgen receptor modulator (SARM) that is an esterified version of the popular compound, RAD 140. The benefits of RAD 140 and RAD 150 are very similar, however, RAD 150 is considered to be the more effective compound following the esterification process. SARMs have been shown to mimic the action of testosterone by effectively binding to androgen receptors. These compounds are becoming more popular and widely available for research purposes as evidence shows SARMs do not elicit negative androgenic side effects, such as increased prostate weight, that are typically associated with testosterone treatment.
Main Research Findings
1) The process of esterification leads to increased potency and improved efficacy of RAD 150 in comparison to its parent compound, RAD 140.
2) SARMs have been shown promote various forms of functional therapy through tissue-selective activation of androgenic signaling
3) Researchers LuRusso et. Al determined treatment with RAD 140 was well-tolerated when administered to women with metastatic breast cancer.
Selected Data
1) As it was previously mentioned, RAD 150 is the esterified form of RAD 140. RAD 140, like most SARMs, is typically known for its ability to promote anabolic activity in bone and muscle tissue. Current research examines the potential of RAD 140 to enhance neuroprotection and stimulate antitumor activity. As an esterified version of RAD 140, RAD 150 has been shown to elicit similar effects as its parent compound with far more potency. The esterification process proceeds by combining an organic acid (RCOOH) and an alcohol (ROH) to form water and an ester (RCOOR) [1].
Furthermore, esterification reactions occur when a primary alcohol is treated with a carboxylic acid in the presence of sulphuric acid. The resulting compound tends to smell sweet and is generally classified as an ester compound. The chemical reaction typically takes place in 5 steps and follows the format:
CH3COOH + CH3CH2COOH → CH3COOCH2CH3
The first step of the esterification mechanism is cation formation. Step 2 of the mechanism delocalized the carbocation by protonating the carboxyl oxygen in order to improve the electrophilic properties of the carbocation. Step 3 transfers a proton to one of the hydroxyl groups to form a sufficient leaving group. Step 5 forms the pi bond by eliminating water and donating an electron pair from the hydroxyl group’s alcohol oxygen atom to the carbon atom. By eliminating water there is no feasible nucleophile available to reverse the reaction. This leads to step 5 which is the ultimate formation of an ester compound. These compounds typically have a pleasant smell, allowing them to be widely used in the perfume, food flavoring, and cosmetics industries. They are also organic compounds typically found in oils and fats that are able to be used and an organic solvent [1].
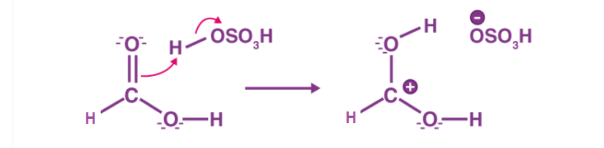
Figure 1: Step 1 of the esterification mechanism
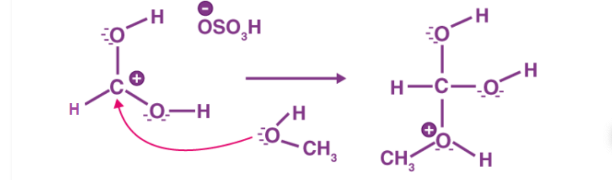
Figure 2: Step 2 of the esterification mechanism
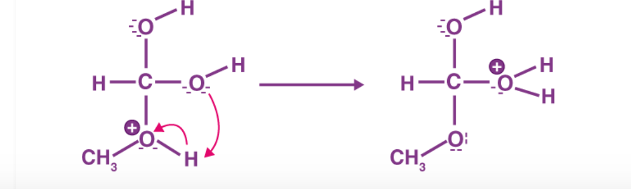
Figure 3: Step 3 of the esterification mechanism
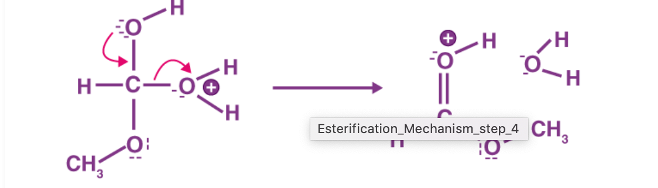
Figure 4: Step 4 of the esterification mechanism
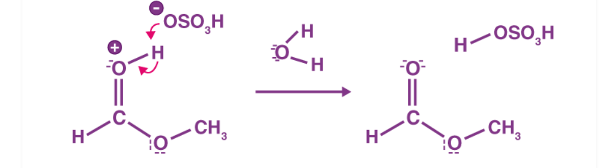
Figure 5: Step 5 of the esterification mechanism
2) The research team of Shalender Bhasin, MD and Ravi Jasuja, PhD. examined the potential of selective androgen receptor modulators (SARMs) to promote various forms of functional therapy. Previous research has reported that SARMs bind to androgen receptors and display tissue-selective activation of androgenic signaling, leading to anabolism in skeletal muscles and bones. The actions of SARMs are compared to testosterone, the major ligand for androgen receptors. Testosterone is often supplemented to men and women of all ages suffering from androgen deficiency and decreased muscle and bone wasting. However, administration of androgenic compounds such as testosterone is often related to many dose-limiting adverse side effects such as prostate dysfunction, edema, and erythrocytosis. On the other hand, SARM administration has been shown to result in similar anabolic activity without the adverse side effects associated with typical androgen treatment [2].
In order to target functional limitations caused by osteoporosis, aging, and chronic disorders, researchers first attempted to develop a SARM with the desired activity profile and tissue selectivity. The second approach included elucidating the mechanisms of action of androgens on skeletal muscles and the prostate in order to identify signaling molecules downstream of the androgen receptors that are capable of activating hypertrophic pathways in skeletal muscles but not the prostate. When observing the structure of SARMs, the compounds can be categorized into two groups: steroidal and nonsteroidal. Steroidal SARMs are synthesized by modifying the chemical structure of testosterone molecules. For example, substitution of 7-alpha alkyl makes testosterone less susceptible to 5-alpha reduction, thus increasing tissue selectivity with respect to the prostate. This results in the increased anabolic activity in the levator ani muscle and a decreased rate of anabolism in the prostate and seminal vesicles [2].
Researchers at the University of Tennessee and Ligand Pharmaceuticals reported early data regarding the discovery of nonsteroidal SARMs. After publication of the initial findings various other structural categories of SARM pharmacophores were examined. These categories included: aryl-propionamide, bicyclic hydantoin, quinolones, tetrahydroquinoline analogs, benzimidazole, imidazolopyrozole, indole, pyrazoline derivatives, azasteroidal derivatives, and aniline, diaryl aniline, and benzoxazepinones derivatives. The first generation of SARMS was developed by manipulating the structure of aryl propionamide analogs, bicalutamide and hydroxyflutamide. This initial discovery led to copious amounts of research dedicated solely to modifying compound structures in order to promote tissue selectivity and further hone in on the beneficial anabolic activity [2].
3) The first phase 1 clinical study regarding the efficacy of RAD140 was conducted by the research team of LoRusso et. Al. The purpose of their experiment was to determine the safety and tolerability of the SARM, as well as to examine the antitumor effects of RAD140 in cases of postmenopausal women with advanced breast cancer. Researchers measured the occurrences of dose-limiting toxicity in order to estimate a recommended dose for expansion (RDE) and maximum tolerated dose (MTD). The secondary goal of the study was to develop a pharmacokinetic profile of the compound. RAD140 was administered in a 3 + 3 dose-escalation phase to subjects diagnosed with inoperable metastatic ER+/HER2- breast cancer. Administration of the SARM was followed by a pharmacokinetic expansion (PKE) [3].
There were approximately 30 patients involved in the first treatment round of RAD 140. The compound was administered to the subjects daily in 50 mg doses in order to proceed with dose-limiting toxicity (DLT) evaluations to determine the MTD and RDE. If patients passed through the first DLT evaluation period without experiencing DLT, they continued on to the second evaluation of the study. When 2 DLTs occurred in 3-6 patients the researchers halted dosage increases. At that point, the MTD was measured as the highest treatment dose where only 1 in every 6 patients experienced only 1 DLT occurrence.
In order to determine the maximum treatment dosage, researchers added a cohort patient group administered a single-dose PK in order to gather information about the safety and tolerability of the compound. Subjects were administered one dose of the hypothesized MTD, followed by 7 days of no treatment. Plasma samples were collected from the subjects during the no treatment period so the researchers could complete PK characterization. After this sample treatment week the cohort group began daily RAD140 treatment at the preliminary MTD [3].
The safety and tolerability of RAD 140 was examined through physical examinations, vital signs, blood tests, ECG, and serum chemistry evaluations. Researchers paid close attention to any changes in serum cardiac troponin levels as an indicator of DLT. The severity of elicited side effects were graded according to the Common Terminology Criteria for Adverse Events. Furthermore, a tumor assessment was performed at baseline and every 8 weeks after, during Cycles 2-12. After Cycle 12 the tumor assessments took place every 3 months.
Additionally, the blood samples were collected to complete the PK characterization and were gathered from the subjects pre- and post-administration of RAD 140. Samples were initially collected Cycle 1 Day 1 at hours 1, 2, 4, 6 ,8, and 24, and were collected again Cycle 1 Day 15 hours 1, 2, 4, 6, and 8. Blood samples pre-dose administration were collected on Day 28 of Cycles 2-6. Pre-dose samples of the PK cohort group were collected at hours 1, 2, 4, 6, 8, 24, 48, 72, 96, and 144 hours after receiving the single-dose of PK [3].
Discussion
1) As an esterified version of RAD 140, RAD 150 is considered a superior version of its parents compound due to its increased potency and improved bioavailability. The esterification process also makes RAD 150 far more stable than RAD 140 suggesting that it is more effective and safe. While the benefits of the two compounds are very similar, there is debate regarding how much stronger RAD 150 is compared to RAD 140. Recent evidence suggests that RAD 150 is approximately 10-15% more potent.
Multiple research-based studies have concluded that SARMs are able to increase muscle mass and reduce fat, however, researchers thought it was important to note that RAD 150 does not reduce fat by targeting fat directly, but rather by raising metabolic rate. RAD 150 increases testosterone leading to improved metabolism and a calorie deficit, ultimately resulting in fat loss. Additionally, evidence suggests that RAD 150 is capable of increasing libido, improving recovery from exercise by increasing protein synthesis, and enhancing physical performance by boosting muscle mass and raising metabolism.
2) The research team of Bhasin and Jasuja were able to achieve selectivity of SARMs by elucidating the mechanism of testosterone’s action on the prostate, as well as how molecules farther downstream were associated with activation of AR signaling in skeletal muscle. Analysis of muscle biopsies collected from male test subjects treated with varying doses of testestore revealed that administration of the compound led to hypertrophy in type I and type II muscle fibers. In relation to testosterone dosage, both type I and type II fibers experienced significant changes in cross-sectional areas. It is important to note that there was no change observed in the absolute number or the relative proportion of type I and type II fibers in response to testosterone administration [2].
Hypertrophy of the skeletal muscle was further examined through observation of muscle satellite cells and the myonuclear number. These variables were assessed through the use of electron microscopy, using direct counting and spatial orientation methods at baseline and after 20 weeks of GnRH agonist and testosterone enanthate treatment. Results reported that absolute and percent satellite cell number was significantly greater than baseline after 20 weeks of the test subjects receiving supraphysiologic doses of testosterone. The observed changes in the number of satellite cells correlated with changes in total and free testosterone levels, indicating that muscle fiber hypertrophy induced by testosterone is correlated with an increase in the number of satellite cells and the myonuclear number.
Recent studies have found that both testosterone and DHT are able to promote association between liganded ARs and beta-catenin, its co-activator. Beta-catenin is stabilized by this interaction and enhances translocation into the nucleus and association with TCF-4, as well as the transcriptional activation of Wnt-target genes. Additionally, Testosterone upregulated the expression of follistatin, resulting in increased muscle mass and decreased fat mass. SMAD 7 is also upregulated by testosterone while TGF-beta-mediated SMAD signaling in TGF-beta target genes is downregulated. The connection between testosterone and follistatin expression indicates that the effects of testosterone are cross-communicated from the WNT pathway to the TGF-beta-SMAD pathway. These results further suggest that candidate molecules located downstream of AR and beta-catenin, such as follistatin, have the potential to mediate the effects of testosterone on the muscle and may provide desired selectivity of anabolism. The discovery of these candidate targets allows for further research to be conducted in order to develop selective anabolic drugs [2].
3) Data collected by researchers LoRusso et. Al allowed the team to conclude that treatment with RAD140 is well-tolerated by postmenopausal subjects diagnosed with metastatic breast cancer. 77.3% of the patients involved in this study reported experiencing side effects ranging in severity from Grade 0 to Grade 4. 31.8% of the patients experienced Grade 3 side effects while 0% of the patients experienced Grade 4 side effects. The more frequent side effects exhibited by the patients were increased weight loss, dehydration, and vomiting, as well as elevated levels of aspartate aminotransferase, alanine aminotransferase, and total bilirubin. It is important to note that 41% of the patients reported serious side effects, however, the researchers determined the serious side effects were not related to this study.
In regards to the occurrence of side effects, the researchers noted that 5 out of 22 patients experienced hypophosphatemia. Out of the 5 cases only 2 were measured at Grade 3 severity, while all 5 cases were deemed capable of reversal. All symptoms of hypophosphatemia in the patients were reversed when RAD140 treatment was temporarily stopped. When treatment resumed at a later date there was no identifiable recurrence of the dose-limiting toxicity [3].
Dose-limiting toxicity (DLT) was observed in 6 out of 17 study-eligible patients. The DLT occurrences were all measured as Grade 3, however, they were deemed capable of reversal. The frequency of DLT occurrences were recorded and used to determine the maximum tolerated dose (MTD) and the recommended dose for expansion (RDE). Results of the study reported that both the MTD and RDE values were approximately 100 mg, when administered once daily.
No significant changes were found when the researchers performed the patients’ physical examinations. Serum levels of 11-deoxycorticosterone (11-DOC) were routinely measured in order to report instances of cardiac inflammation or changes in the hormone biosynthetic pathway. None of the treatments administered to the patients resulted in changes in mean and median 11-DOC levels. In addition to 11-DOC the researchers recorded the levels of cardiac troponin and troponin T found in the plasma. The baseline measurements were used as a way to grade severity ranging from baseline to worst post-baseline value. Troponin T levels were recorded at Grade 0 at baseline values, and only increased slightly to Grade 1 following treatment [3].
Any cardiac side effects elicited by RAD140 were only found in 18.2% of test subjects. The primary side effects reported were Grade 1 palpitations, Grade 2 atrial flutter and atrial fibrillation, and Grade 3 sinus tachycardia and ventricular failure. Patients experiencing Grade 3 tachycardia were released from the study for other treatment purposes. However, the researchers were able to conclude that any of the cardiac side effects seen in the patients were not related to treatment with RAD140. Furthermore, the research team observed that there were no adverse androgenic side effects elicited by the SARM; Grade 1 weight gain, acne, and hair growth were the only reported side effects experienced by the patients [3].
Finally, the pharmacokinetic report developed by the research team reported that the initial treatment groups experienced an elevation in plasma concentration for approximately 24 hours after treatment with RAD140. When RAD140 was administered to the additional, single-dose PK cohort group, serum levels of the SARM increased rapidly, followed by a steady decline resulting in secondary and tertiary peaks. The PK evaluation led the researchers to conclude that RAD140 has an acceptable safety profile and is capable of promoting antitumor activity, specifically in pretreated postmenopausal women diagnosed with metastatic breast cancer.
Disclaimer
**LAB USE ONLY**
*This information is for educational purposes only and does not constitute medical advice. THE PRODUCTS DESCRIBED HEREIN ARE FOR RESEARCH USE ONLY. All clinical research must be conducted with oversight from the appropriate Institutional Review Board (IRB). All preclinical research must be conducted with oversight from the appropriate Institutional Animal Care and Use Committee (IACUC) following the guidelines of the Animal Welfare Act (AWA).
Citations
[1] “Esterification (Alcohol & Carboxylic acid) – Reactions Mechanism & Uses with Videos.” Byju’s, https://byjus.com/chemistry/esterification/. Accessed 31 May 2023.
[2] Bhasin S, Jasuja R. Selective androgen receptor modulators as function promoting therapies. Curr Opin Clin Nutr Metab Care. 2009 May;12(3):232-40. doi: 10.1097/MCO.0b013e32832a3d79. PMID: 19357508; PMCID: PMC2907129.
[3] LoRusso P, Hamilton E, Ma C, Vidula N, Bagley RG, Troy S, Annett M, Yu Z, Conlan MG, Weise A. A First-in-Human Phase 1 Study of a Novel Selective Androgen Receptor Modulator (SARM), RAD140, in ER+/HER2- Metastatic Breast Cancer. Clin Breast Cancer. 2022 Jan;22(1):67-77. doi: 10.1016/j.clbc.2021.08.003. Epub 2021 Aug 20. PMID: 34565686.
RAD-150 SARM is sold for laboratory research use only. Terms of sale apply. Not for human consumption, nor medical, veterinary, or household uses. Please familiarize yourself with our Terms & Conditions prior to ordering.




| File Name | View/Download |
| 02-10-2023-Umbrella-Labs-RAD-150-TLB150-Certificate-Of-Analysis-COA.pdf |
VIEW CERTIFICATES OF ANALYSIS (COA)
Additional information
| Weight | 2 oz |
|---|---|
| Dimensions | 3 × 3 × 5 in |
| CAS Number |



















