5-ALPHA-HYDROXY LAXOGENIN (SARM SUPPORT) – 100MG/ML – 30ML/60ML BOTTLE
$46.99 – $92.98
5-ALPHA-HYDROXY LAXOGENIN is sold for laboratory research use only. Terms of sale apply. Not for human consumption, nor medical, veterinary, or household uses. Please familiarize yourself with our Terms & Conditions prior to ordering.
Also Available In:
![]() Powder Option >>
Powder Option >>
![]() Gel Option >>
Gel Option >>
- Description
- Additional information
Description
5-Alpha-Hydroxy Laxogenin Liquid
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
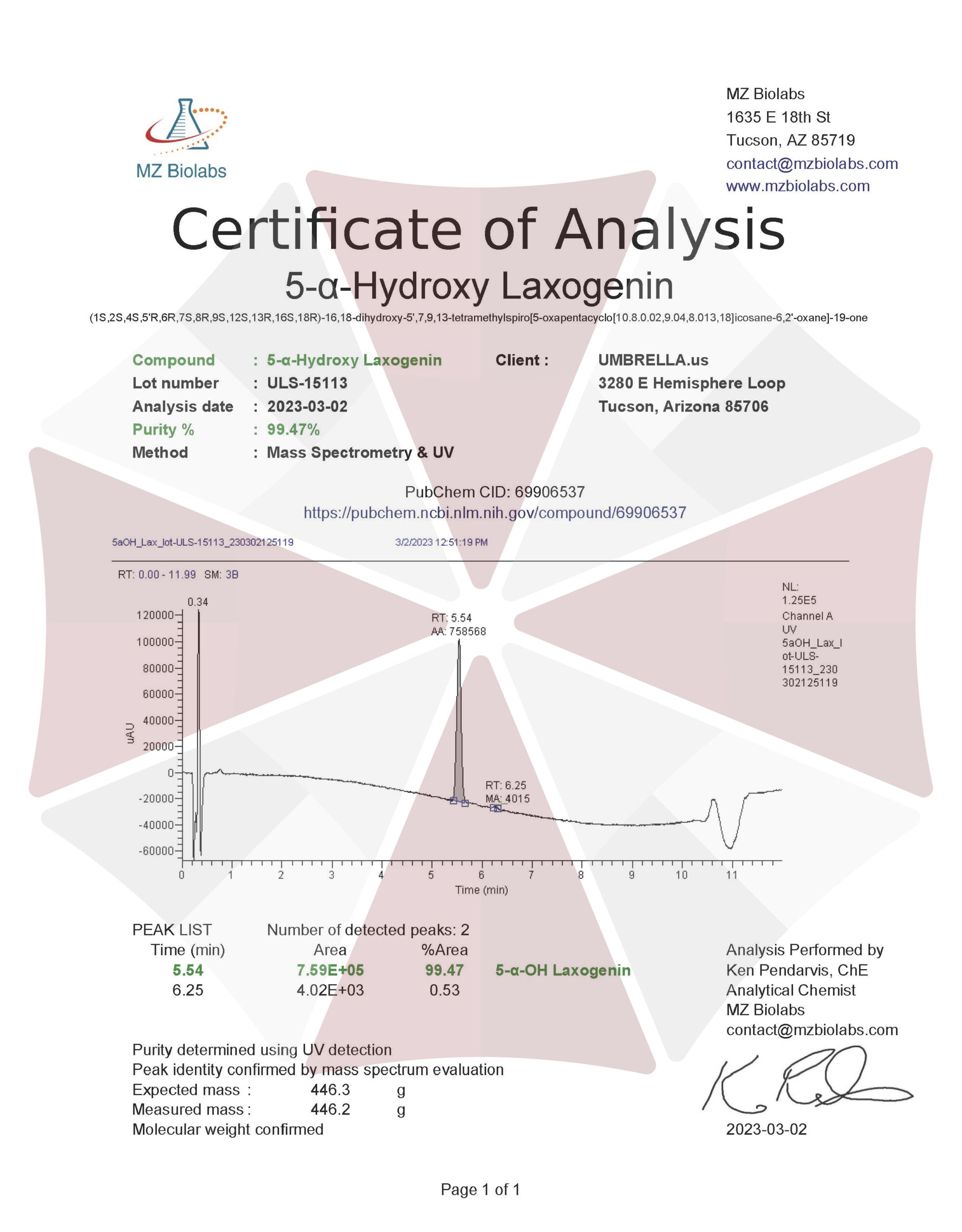
| CAS Number | 56786-63-1 |
| Other Names | 5alpha-Hydroxy laxogenin, 5-alpha-Hydroxy-laxogenin, Biobras 16, Irgalite Yellow wgp, Brassinosteroid BB 16, Di-31, 25R,5alpha-Spirostan-3beta,5-diol-6-one, 844KE20WT5 |
| IUPAC Name | (1S,2S,4S,5’R,6R,7S,8R,9S,12S,13R,16S,18R)-16,18-dihydroxy-5′,7,9,13-tetramethylspiro[5-oxapentacyclo[10.8.0.02,9.04,8.013,18]icosane-6,2′-oxane]-19-one |
| Molecular Formula | C₂₇H₄₂O₅ |
| Molecular Weight | 446.6 |
| Purity | ≥99% Pure (LC-MS) |
| Liquid Availability | |
| Powder Availability | |
| Gel Availability | |
| Storage | Store in cool dry environment, away from direct sunlight. |
| Terms | All products are for laboratory developmental research USE ONLY. Products are not for human consumption. |
What is 5-Alpha-Hydroxy Laxogenin?
Synthetically derived from laxogenin, 5-alpha hydroxy laxogenin is another popular compound that has been shown to improve muscle growth. 5-alpha hydroxy laxogenin is typically derived from the plant Smilax Sieboldii, however, when used for research purposes the compound is produced from the plant-based raw material, diosgenin. Diosgenin is typically used in pharmaceutical prep for synthetic steroidal drugs.
Brassinosteroids are considered a phytohormone that plays a role in plant growth and development. Additionally, brassinosteroids are incredibly similar to various animal hormones in the way that they affect various aspects of growth and synthesization such as cell elongation, cell division, immunity, and reproduction [1].
While research on 5-alpha hydroxy laxogenin is limited, reported effects state that the compound works by increasing the rate of protein synthesis while simultaneously decreasing the rate at which protein breaks down. The net positive effect that 5-alpha hydroxy laxogenin has on protein synthesis indicates that supplementation will ultimately lead to an increase in lean muscle mass.
Main Research Findings
1) Brassinosteroids have the potential to enhance lean body mass and androgenic activity as well as significantly increase the amount of muscle fibers present.
2) Results collected from in vitro bioassays emphasize the ability of 5-alpha-hydroxy-laxogenin to enhance androgenic potential and anabolic activity.
Selected Data
1) As it was previously mentioned brassinosteroids are plant-derived polyhydroxylated derivatives of 5a-cholestane. The research team of Esposito et. Al examined how 28-Homobrassinolide (HB) has the potential to promote growth and protein synthesis while inhibiting protein degrading in L6 rat skeletal muscle cells. HB was initially purchased from Waterstone Technology. Phenylalanine was obtained from GE Healthcare while all reagents and enzymes used for quantitative PCR were collected from Stratagene and Applied Biosystems. Rat L6 skeletal muscle cell line CRL-1458 was obtained from the American Type Culture Collection [2].
In order to measure the rate of protein synthesis in cells treated with HB, differentiated myotubes were washed with serum-free DMEM and treated three times with either a vehicle composed of 0.1% ethanol, increasing concentrations of HB, or 6.5 nM of IGF-1 as a positive control. The experimental compounds were added to serum-free medium and incubated for 4 hours. Incubation was stopped by placing the cells on ice, discarding the medium, and washing the cells with PBS to remove the nonincorporated trace. The proteins were then precipitated with 5% trichloroacetic acid and dissolved in 0.5 N NaOH while specific radioactivity of protein-bound phenylalanine was quantified using the liquid scintillation counter. Samples were then normalized to milligrams of total protein and determined by BCA protein assay.
When investigating the effects of HB on protein degradation, the research team utilized differentiated myotubes, as previously described. The myotubes were incubated for 16 hours to allow labeling of cellular protein followed by washing with PBS to remove the nonincorporated trace. The cells were then treated for 4 hours with either a vehicle composed of 0.1% ethanol, increasing concentrations of HB, or 10 nM of insulin in serum-free medium. Incubation was stopped by placing the cells on ice, and 5% trichloroacetic acid was used to precipitate the protein in the medium [2].
All animal-based experiments were performed according to the procedures approved by the Rutgers Institutional Animal Care and Use Committee in an Association for Assessment and Accreditation of Laboratory Animal Care. The animals were housed in individual cages and maintained at a constant temperature and strict 12-hour light/12-hour dark schedule. The animals adapted for 7 days followed by random distribution into groups according to body weight prior to dosing.
The first protocol of the study included three groups of Wistar rats fed a normal diet containing 23.9% protein, 10.7% fat, 5.1% fiber, and 58.7% carbohydrates, resulting in 4.61 kcal/g energy value. The subjects were gavaged daily for 24 days with 1 ml of a vehicle composed of 5% DMSO in corn oil, or 20 or 60 mg/kg body weight of HB. Every 2 days the researchers recorded the body weight of each animal as well as the total amount of food consumed over the course of the experimental period. At the end of the experimental blood was collected via heart puncture after inhalation of CO2. Body composition of the test subjects was examined prior to necropsy; at necropsy the tissue weights were measured and recorded while tissue samples were collected by snap-freezing in liquid nitrogen for further examination. Protocol 2 included three groups of Wistar rats fed a high-protein diet containing 39.4% protein, 10.0% fat, 4.3% fiber, and 37.0% carbohydrates resulting in 3.93 kcal/g energy value. The subjects were gavaged daily for 24 days with 1 ml of a vehicle composed of 5% DMSO in corn oil, or 20 or 60 mg/kg body weight of HB. Further procedures and measurements followed the protocol 1 setup [2].
Protocol 3 included four-week-old sham-operated Wistar rats that were subjected to 10 day Hershberger assay. The experimental groups included: sham, ORX (vehicle), ORX (20 mg/kg of HB orally), ORX (60 mg/kg of HB orally), and ORX (0.4 mg/kg of testosterone propionate subcutaneously, acting as a positive control). Procedures and measurements followed the protocol 1 setup, however no body composition measurements were recorded. The control animals and the animals receiving 60 mg/kg of HB had limb grip strength measured using a digital force gauge. Three trials were performed on each animal and the significance of the results was determined using Student’s t test. Additionally, the gastrocnemius muscle, ventral prostate, seminal vesicles, bulbocavernosus/levator ani muscle complex, glans penis, and Cowper’s gland were extracted post-euthanasia and weighed. Finally, protocol 4 included four-week-old sham-operated or ORX Wistar rats that were subject to 10 day Hershberger assay. The experimental groups included sham, ORX (vehicle), ORX (0.4 mg/kg of HB subcutaneously), ORX (4 mg/kg of HB subcutaneously), and ORX (0.4 mg/kg of testosterone propionate subcutaneously to act as a positive control) [2].
Body composition of the test subjects was assessed through the use of DEXA scanning using Lunar Prodigy total body scanner that used Encore small animal body software. Quality control was performed on a daily basis and the coefficient of variation for total body, lean mass, and bone mineral content was measured in 5 rats, 3 times each. Furthermore, the muscle samples for the histochemical analysis were taken from the mixed-fiber gastrocnemius muscle. Serial transverse cryosections were prepared from each muscle and analyzed for myofibrillar adenosine triphosphate (mATPase) histochemistry, following alkaline preincubation. Fiber cross section area and enzyme activity levels were determined by examining digitized images of the muscle cross-sections [2].
2) The research team of Beer et. Al examined the androgenic properties of 5-alpha-hydroxy-laxogenin through two in vitro bioassays: a yeast androgen screen and a reporter gene assay in a human prostate cell line. Dihydrotestosterone, bicalutamide, and hydroxyflutamide were all provided by Sigma-Aldrich in Munich Germany while 5-alpha-hydroxy-laxogenin was purchased from Biomol, located in Hamburg, Germany. DImethyl sulfoxide (DMSO) was provided by Carl Roth; all test compounds were dissolved in DMSO. DMEM, FBS, and penicillin/streptomycin were supplied by BioWest and luciferase assay systems were obtained by Promega. Human PC3(AR2) cells as well as the reporter plasmid encoding the luciferase gene in the mouse mammary tumor virus, were provided by Dr. Aria Baniahmad.
The first in vitro study performed was a yeast androgen screen that was performed using a Saccharomyces cerevisiae strain that was previously transfected with a human androgen receptor (AR) construct as well as a reporter plasmid carrying the beta-galactosidase encoding LacZ under control of androgen responsive elements. The yeast cells used in the study were treated with either DMSO as a solvent control, DHT as a positive control, or dilutions of 5-alpha-hydroxy-laxogenin. In order to examine antagonistic properties, 5-alpha-hydroxy-laxogenin was co-incubated with flutamide with beta-galactosidase activity was measured through the hydrolysis of chloro-phenol red-beta-D-galactopyranoside. All experimental treatments were performed independent from each other and Student’s t test was used to determine statistical significance [3].
Human PC3(AR)2 cells were maintained in DMEM/F12 and supplemented with 1% penicillin/streptomycin, 0.25 mg/mL G418, and 10% FBS. The cells were then cultivated in DMEM/F12 containing 1% penicillin/streptomycin, and 5% dextran-coated charcoal-treated FBS. 70,000 cells were seeded in a 24-well plate and transfected with the reporter plasmid mmTV-luc. PC3(AR)2 cells were treated with 0.1% DMSA as a solvent control, DHT as a positive control, or 5-alpha-hydroxy-laxogenin. The cells were then co-incubated with bicalutamide and 5-alpha-hydroxy-laxogenin in order to examine the antagonistic effects of the experimental compounds. Luciferase activity and quantified protein concentration was measured using the bicinchoninic acid assay with BSA as standard protein. Additionally, Relative Luminescence Units (RLU) were calculated by normalizing the luminescence with the protein concentrations. Three independent cell culture experiments were performed and one-way ANOVA followed by Bonferroni’s post hoc test were used to assess statistical significance for this portion of the study [3].
Discussion
1) The results of the study conducted by Esposito et. Al initially reported that HB showed no toxicity to the fully differentiated L6 rat skeletal myotubes. In order to determine the effects of HB on protein synthesis, radiolabeled phenylalanine was incorporated and the response was assessed by the researchers. The cell samples were treated with different doses of HB for 4 hours; the lower concentration of 1 uM HB was shown to increase protein synthesis by 12.4 ± 2.3% above control levels. The response approached saturation when the cell samples were treated with concentrations of HB between 10 and 20 uM; protein synthesis increased by 34.9 ± 3.1 and 36.9 ± 2.9%, respectively. A 6.5 nM concentration of IGF-1 was used as a positive control in this portion of the study, resulting in a 42.5 ± 4.5% increase in protein synthesis. The cell samples treated with the higher concentrations of HB were shown to be less effective. A 1- to 24-hour study was performed next in order to investigate the time-dependent effects of HB on protein synthesis. The researchers observed a time-dependent increase in protein synthesis in response to HB. Protein synthesis induced by HB peaked at 3 hours and began to decrease at 4 hours; a similar kinetic pattern was seen in the cells treated with IGF-1 [2].
In terms of protein degradation, results of the study reported that IGF-1, used as a positive control in this study, has an anabolic effect on protein synthesis and an anticatabolic effect on protein degradation in muscle tissue, in a manner similar to insulin. Protein synthesis is more responsive to infusion with IGF-1 than to infusion with insulin, however, insulin is capable of inhibiting protein degradation. The researchers monitored degradation of the protein labels with phenylalanine through the release of acid-soluble radioactivity into the medium. When the cell samples were treated with concentrations of HB ranging from 0.3-20 uM, protein degradation was inhibited in a dose-dependent manner. Overall activity plateaued between 3 and 10 uM concentration of HB. A 1 uM concentration of HB was shown to decrease protein degradation by 8.2 ± 0.6% above control levels. At a higher concentration of 10 uM of HB, protein degradation was decreased by 9.5 ± 0.9% above control levels. 10 nM of insulin was used as a positive control and was shown to inhibit protein degradation by 13.0 ± 1.6%. A 1- to 4-hour study was performed next in order to investigate the time-dependent effects of HB on protein synthesis. The researchers observed a time-dependent increase in protein synthesis in response to both HB and insulin that reached a plateau after 3 hours [2].
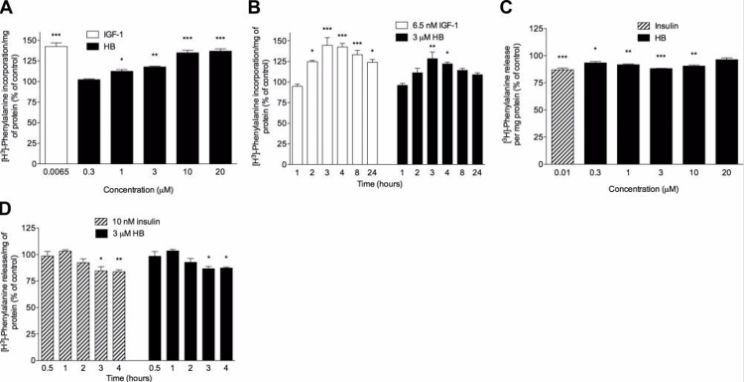
Figure 1: Concentration- and time-dependent effects of HB on protein synthesis and degradation. A) cells incubated for 4 hours with phenylalanine, HB, or IGF-1, B) time-dependent effects of HB treatment on protein synthesis in cells treated with HB or IGF-1, C) dose-dependent effects on protein degradation, D) HB time-course study of protein degradation.
When examining the effects of HB on body composition in Wistar rats, the test subjects were administered daily doses of 20 and 60 mg/kg of HB (HB20 and HB60, respectively) over the course of 24 days. At the end of treatment the HB20 and HB60 treatment groups experienced an 18.3% and 26.6% respective increase in total body weight gain relative to initial body weight. When adjusted for body weight there were no significant changes in food intake among the treatment groups. This indicates that the increase in body weight in the HB groups was not caused by increased food intake. DEXA scans found that body composition of the test subjects reflected an increase in lean body mass; the HB20 and HB60 groups experienced a 7.0% and 14.2% increase in lean body mass as well as a 3.9% and 4.9% decrease in fat mass, respectively. These results allowed the researchers to conclude that the reported weight gain was due to a significant increase in lean body mass. Additionally, treatment with HB increased muscle mass of the gastrocnemius and slightly improved total body bone mineral content.
The anabolic effects of HB were examined in rats that were fed a high-protein diet rather than a standard diet. The diet of the test subjects consisted of approximately 40% protein, in addition to oral administration with 20 and 60 mg/kg of HB, daily for 24 days. In comparison, the control animals that were fed a high-protein diet ended up consuming less food and gaining less food than the control animals that were fed a standard diet. Furthermore, the high-protein diet was capable of enhancing the stimulatory effects of the 20 mg/kg dose of HB on body weight. However, there were no HB-related increases in food intake in either the control or experimental test subjects. Other than the recorded changes in body weight there were no additional changes in body composition or blood biochemistry that could be linked to HB treatment or a high-protein diet [2].
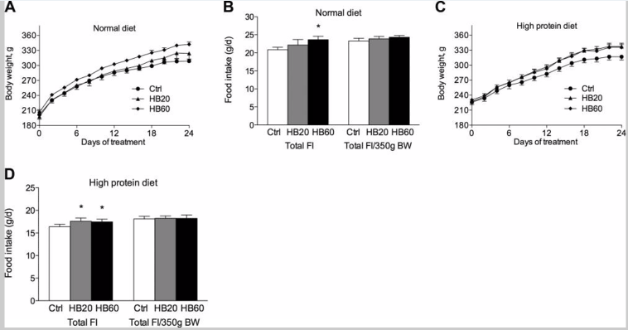
Figure 2: Effect of HB on body weight gain and food intake in rats fed (A,B) a normal diet or (C,D) a high-protein diet
The next portion of the study compared the ability of HB and testosterone propionate to restore androgen-dependent tissues after androgen deprivation was induced through surgical castration. Androgen deprivation led to a significant decrease in the size of the prostate, seminal vesicles, bulbocavernosus/levator ani muscle complex, glans penis, and Cowper’s gland; these organs decreased by 8.6, 6.5, 23.9, 54.6, and 40.5%, respectively, in comparison to the sham-operated animals. Testosterone propionate was injected in doses of 0.4 mg/kg resulting in a 3- to 8-fold increase in androgen sensitive tissues. However, testosterone propionate failed to restore the weight of the ventral prostate, seminal vesicles, and the bulbocavernosus/levator ani muscle complex.
In comparison to testosterone propionate, 10 days or oral administration with 20 and 60 mg/kg of HB did not prevent the loss of androgen sensitive tissue that was related to surgical castration. However, there was a dose-dependent increase in the glans penis following HB treatment. HB also increased the weight of the bulbocavernosus/levator ani muscle complex that is typically used as the skeletal muscle biomarker of anabolic activity. The two previously mentioned organs were the only androgen-sensitive tissues that displayed a significant change in weight in comparison to the control animals [2].
2) The study conducted by Beer et. Al performed two in vitro assays in order to examine the androgenic properties of 5-alpha-hydroxy-laxogenin. The SARM was shown to be able to trans-activate the androgen receptors in human prostate cells in a dose-dependent manner. Additionally, a biphasic response was observed with antagonist properties at low concentrations and agonist properties at high concentrations. The androgenic properties of the 5-alpha-hydroxy-laxogenin suggest that further research should be conducted regarding the anabolic properties of the compound.
The first in vitro examination that was performed was a yeast androgen screen that used a Saccharomyces cerevisiae strain transfected with a human AR construct and reporter plasmid carrying the beta-galactosidase encoding the LacZ gene. The results of the yeast androgen screen reported that the addition of DHT stimulated the reporter gene expression in a dose-dependent manner. However, the reporter gene beta-galactosidase was not activated by any of the concentrations of 5-alpha-hydroxy-laxogenin administered to the cells [3].
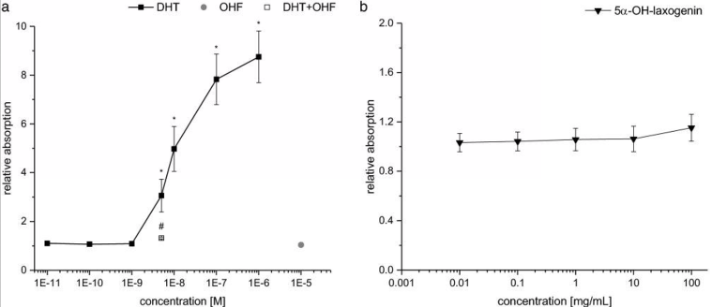
Figure 3: Androgenic dose response in the yeast androgen screen A) DHT as a positive control, B) 5-alpha-hydroxy-laxogenin
On the other hand, 5-alpha-hydroxy-laxogenin was able to induce luciferase expression in human PC3(AR)2 cells in a dose-dependent manner. The SARM elicited biphasic effects with lower doses acting as an antagonist and higher doses acting as an agonist. Bicalutamide, a non-steroidal AR antagonist, was co-incubated with 5-alpha-hydroxy-laxogenin resulting in the antagonism of luciferase activity. These results suggest that 5-alpha-hydroxy-laxogenin has the potential to bind to human AR and acts as an agonist in the PC3(AR)2 cells. The research team hypothesized that the discrepancy between the results of the two in vitro examinations could potentially be due to the different cofactor pattern in yeast and mammalian cells, as well as the additional yeast cell wall. That being said, the researchers thought it was important to note that false negative results of the yeast androgen screen are possible [3].
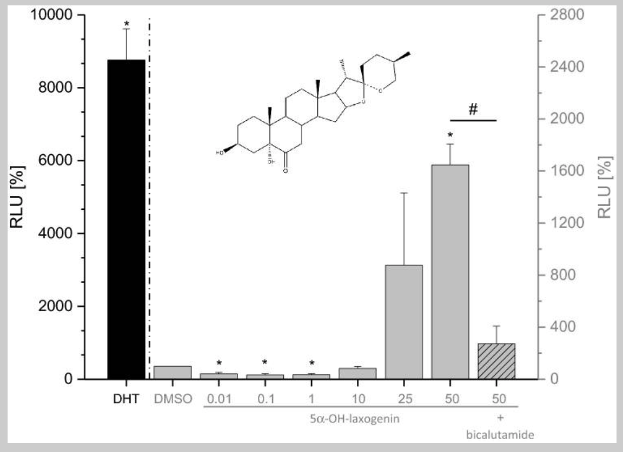
Figure 4: Androgen receptor transactivation in human PC3(AR)2 cells.
Disclaimer
**LAB USE ONLY**
*This information is for educational purposes only and does not constitute medical advice. THE PRODUCTS DESCRIBED HEREIN ARE FOR RESEARCH USE ONLY. All clinical research must be conducted with oversight from the appropriate Institutional Review Board (IRB). All preclinical research must be conducted with oversight from the appropriate Institutional Animal Care and Use Committee (IACUC) following the guidelines of the Animal Welfare Act (AWA).
Citations
[1] Tang, J., Han, Z. & Chai, J. Q&A: what are brassinosteroids and how do they act in plants?. BMC Biol 14, 113 (2016). https://doi.org/10.1186/s12915-016-0340-8
[2] Esposito D, Komarnytsky S, Shapses S, Raskin I. Anabolic effect of plant brassinosteroid. FASEB J. 2011 Oct;25(10):3708-19. doi: 10.1096/fj.11-181271. Epub 2011 Jul 11. PMID: 21746867; PMCID: PMC3177571.
[3] Beer C, Keiler AM. Androgenic properties of the dietary supplement 5α-hydroxy-laxogenin. Arch Toxicol. 2022 Jul;96(7):2139-2142. doi: 10.1007/s00204-022-03283-5. Epub 2022 Mar 28. PMID: 35344071; PMCID: PMC9151512.
5-ALPHA-HYDROXY LAXOGENIN is sold for laboratory research use only. Terms of sale apply. Not for human consumption, nor medical, veterinary, or household uses. Please familiarize yourself with our Terms & Conditions prior to ordering.






| File Name | View/Download |
| 03-02-2023-Umbrella-Labs-5-Alpha-Hydroxy-Laxogenin-Certificate-Of-Analysis-COA.pdf |
VIEW CERTIFICATES OF ANALYSIS (COA)
Additional information
| Weight | 4 oz |
|---|---|
| Dimensions | 3 × 3 × 5 in |
| PubChem CID | |
| Size |