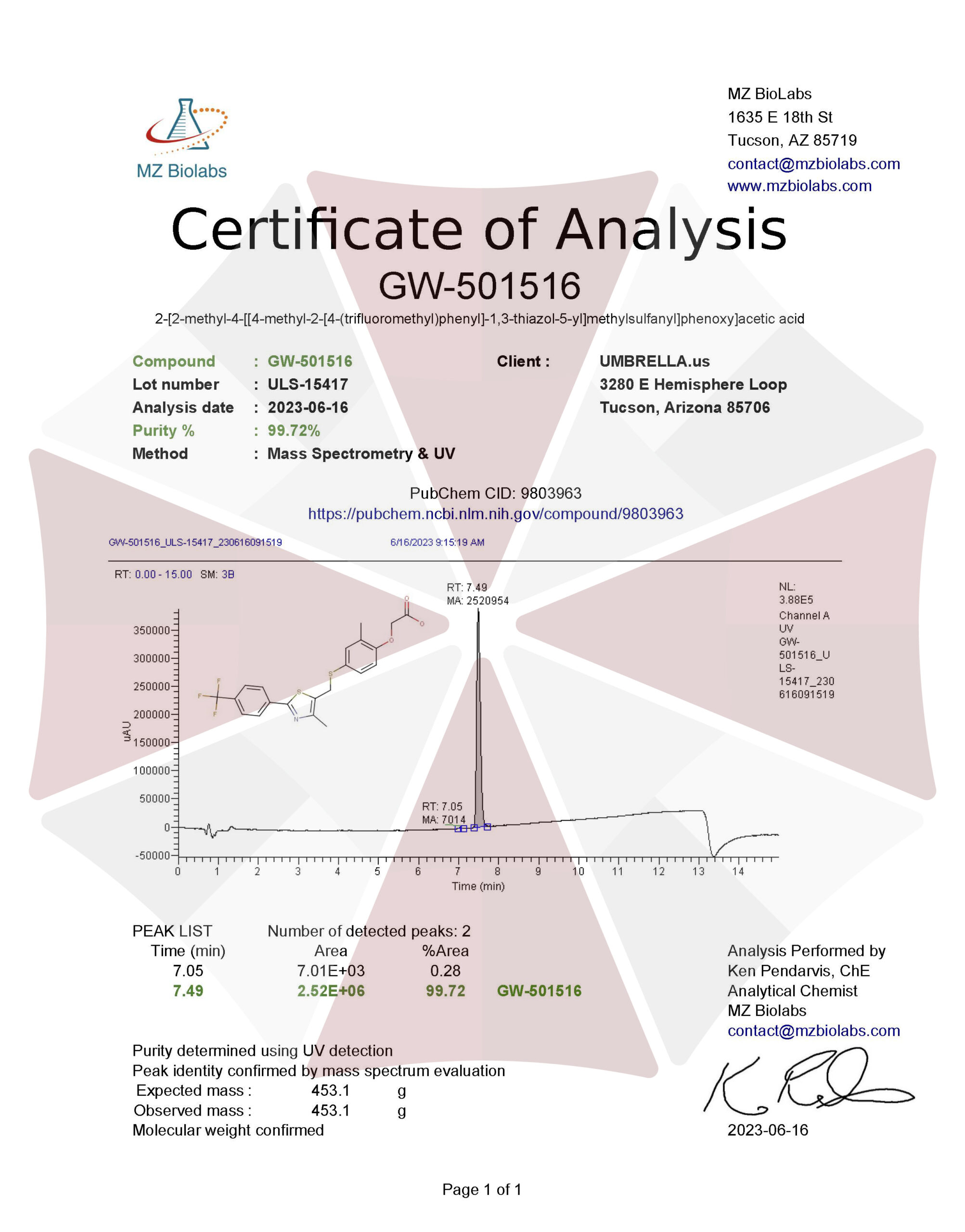
GW-501516 CARDARINE POWDER – 1000MG / 1 GRAM
$70.99
GW-501516 is sold for laboratory research use only. Terms of sale apply. Not for human consumption, nor medical, veterinary, or household uses. Please familiarize yourself with our Terms & Conditions prior to ordering.
*Includes:
- One (~10mg – 15mg) Red Micro Scoop
Also Available In:
![]() Liquid Option >>
Liquid Option >>
![]() Gel Option >>
Gel Option >>
- Description
- Additional information
Description
GW-501516 Cardarine Powder
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
| CAS Number | 317318-70-0 |
| Other Names | GW501516, GW 501516, GW-501516, Cardarine, Endurobol, GSK-516, GW1516, GW 1516, UNII-7I2HA1NU22 |
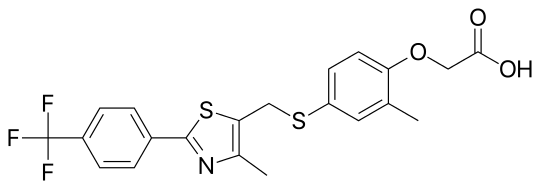
| IUPAC Name | 2-[2-methyl-4-[[4-methyl-2-[4-(trifluoromethyl)phenyl]-1,3-thiazol-5-yl]methylsulfanyl]phenoxy]acetic acid |
| Molecular Formula | C₂₁H₁₈F₃NO₃S₂ |
| Molecular Weight | 453.5 |
| Purity | ≥99% Pure (LC-MS) |
| Liquid Availability |
|
| Powder Availability | |
| Gel Availability | |
| Storage | Store in cool dry environment, away from direct sunlight. |
| Terms | All products are for laboratory developmental research USE ONLY. Products are not for human consumption. |
*Includes:
- One (~10mg – 15mg) Red Micro Scoop
GW-501516 SARM for sale is an orally-available, non-steroidal SARM-like compound that animal studies demonstrate functions like an “exercise mimetic.” It has delivered fantastic results in multiple animal studies, and it continues to be investigated in high-impact research. It binds to and activates the PPARδ receptor, which is a crucial hormone-binding effector molecule whose activity is increased by exercise. This function led to GW-501516 becoming a compound that rose to prominence in the late 2000’s and is still being actively researched for its ability to increase fat-burning capacity, promote synthesis of type I muscle fibers, and reduce cancer burden.

CAS: 317318-70-0
GW-501516 SARM studies were famously pioneered by world-class biologist Ronald Evans at the Salk Institute in La Jolla, CA. He regarded GW-501516 as the long sought-after molecule to confer metabolic protection, calling it “a multifaceted therapeutic for metabolic syndrome with the potential to control weight gain, enhance physical endurance, improve insulin sensitivity, and ameliorate atherosclerosis” [1]. For the better part of decade, many people believed that GW-501516 SARM was permanently derailed by unfortunate animal data that resulted from improper dosing. However, a series of recent high-impact cancer research studies, from 2016 through 2019, show that GW-501516 actually has definitive anticancer properties [2]–[4].
Main Research Findings for GW-501516:
1. Enhances running endurance by 80% or more [5]–[7]
2. Decreases serum triglycerides in normal-weight humans who consume calorie-dense diets [8]
3. Boosts fatty-acid oxidation [9], [10]
4. Reduces obesity and insulin resistance [7]
5. Prevents vascular dysfunction by improving folate signaling [11]
6. Increases immune system protection from cancer [3]
7. Promotes cancer cell death (apoptosis) [2]
8. Reduces cancer-associated inflammation [4]
9. Inhibits neurotoxicity [12]
10. Protects against atherosclerosis [13]
11. Promotes stem cell maintenance in bone marrow [14]
Selected Preclinical Data
1. “Activation PPARδ [by GW-501516] induces fatty acid β-oxidation in skeletal muscle and attenuates metabolic syndrome” [9]
In this study, the authors defined the role of peroxisome proliferator-activated receptor β/δ (PPARδ) in metabolic homeostasis by using subtype selective activators. Analysis of rat L6 myotubes (skeletal muscle cells) treated with the PPARδ activator, GW-501516, by gene expression studies revealed that PPARδ controls fatty acid oxidation by regulating genes involved in fatty acid transport, β-oxidation, and mitochondrial respiration. Similar PPARδ-mediated gene activation was observed in the skeletal muscle of GW-501516-treated animals. Accordingly, GW-501516 treatment caused fatty acid β-oxidation in L6 myotubes as well as in skeletal muscles. Aniamls fed GW-501516 along with a calorie-dense diet showed reducded obesity and improved insulin resistance, effects that were accompanied by enhanced metabolic rate and fatty acid β-oxidation, proliferation of mitochondria (the powerhouses of cells), and a significant reduction of lipid fat droplets in skeletal muscles. Despite a modest body weight change relative to placebo-treated mice, GW-501516 treatment also improved diabetes as revealed by the decrease in blood glucose and blood insulin levels in genetically obese animals. These results strongly suggest that PPARδ activated by GW-501516 is pivotal to control the program for fatty acid oxidation in the skeletal muscle, thereby protecting against body fat increases and insulin resistance.
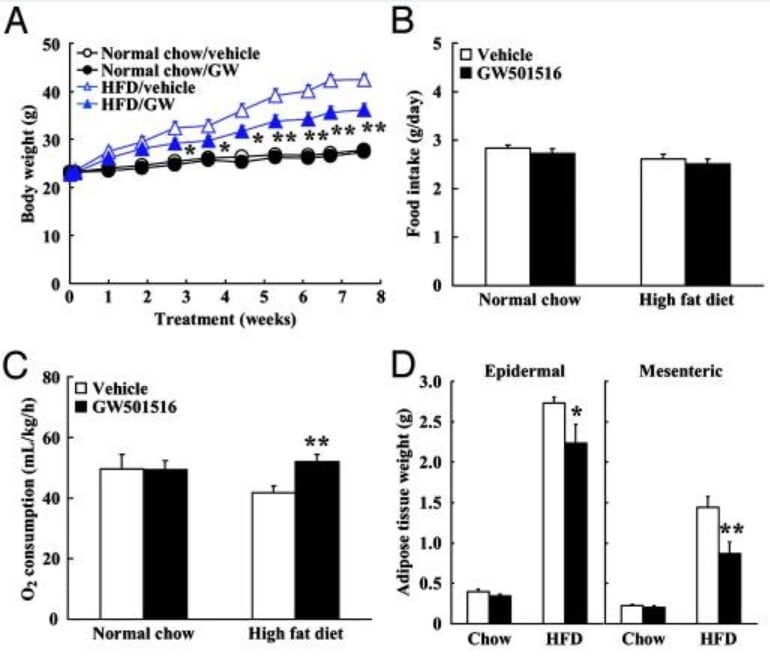
Fig. 1. GW-501516 SARM supplementation prevents fat accumulation (panel A) from a high fat diet (HFD) without changing food intake (panel B). GW-501516 SARM also increases oxygen consumption used for fat burning (panel C) and reduces adipose fat tissue weight in both the epidermis and mesenteric (internal) organs (panel D).
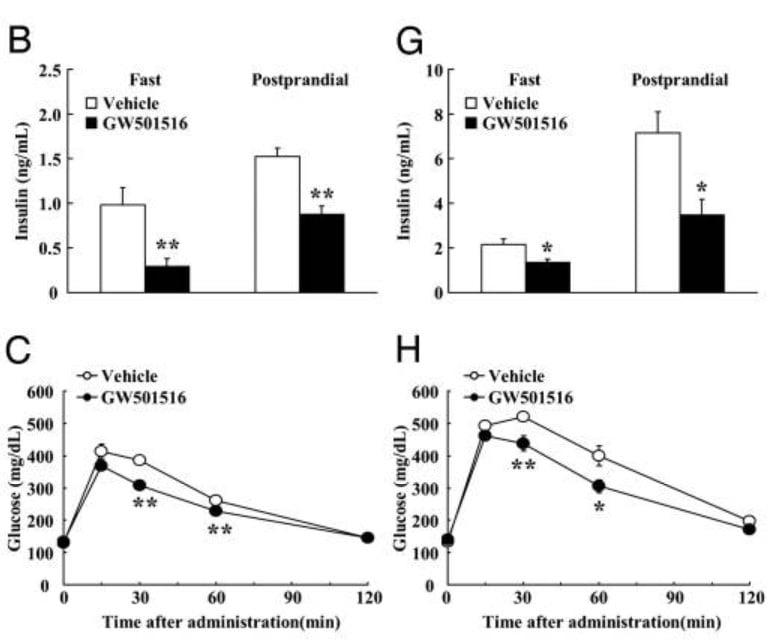
Fig. 2. GW-501516 reduces circulating insulin levels in both fasted animals and after feeding (postprandial). GW-501516 SARM also reduces glucose spike in blood after feeding.
Discussion
In this study, using GW-501516 SARM as a PPARδ agonist, the authors reveal a crucial role of PPARδ in skeletal muscle, which adds to the previously reported role of PPARδ in adipose fat tissues. They discovered that GW-501516 stimulates almost the entire gene expression response for fatty acid use and energy expenditure in skeletal muscle. This increase in fatty acid β-oxidation and energy expenditure in skeletal muscle results in a protective effect of GW-501516 on the development of obesity in animals on a high calorie diet.
Administration of GW-501516 SARM to both high calorie diet and obese animals also significantly improved glucose tolerance and insulin sensitivity. The mechanism by which GW-501516 improves insulin sensitivity is not fully clear yet, but the substantial reduction in fat content in both skeletal muscle and liver, consequent to enhanced energy expenditure, can partially account for the enhanced insulin sensitivity. Accumulation of excess fat in skeletal muscle or liver disrupts insulin signaling. In skeletal muscle, this disruption has already been tied to impaired function of crucial insulin-linked genes. The reduction of visceral obesity observed may also explain why the improvement of insulin resistance occurred through a reduction in inflammatory hormones. These findings alsoreveal that GW-501516 has a potential positive effect on glucose-induced insulin secretion and raises the possibility that it may prevent or delay pancreatic islet cell loss which leads to diabetes [15], [16]. In fact, GW-501516 SARM treatment of pancreatic islet cells isolated from mice on normal diets significantly stimulated glucose-induced insulin release in vitro.
During starvation, glucose uptake and oxidation are rapidly reduced in muscle, which shifts to use free fatty acids and ketone bodies instead. It is especially interesting to note that the alterations in gene expression caused by GW-501516 in skeletal cells in vitro and in skeletal muscle in vivo were very similar to the gene expression changes observed in skeletal muscle upon dietary fasting. On challenges such as cold exposure, fasting, or prolonged exercise, far burning in white fat occurs. These free fatty acids are then released into the circulation are taken up by thermogenic tissues, such as brown fat and skeletal muscle, where they serve as fuel for thermogenesis (heat generation). Thus, these data support the conclusion that one of the physiological roles of PPARδ activated by GW-501516 is to direct metabolism away from utilization of glucose and towards utilization of fats instead, thus causing loss of body fat. It is conceivable that the free fatty acids released from fat tissues upon fasting or exercise provide PPARδ with natural activators that function identically to GW-501516 SARM to stimulate fatty acid oxidation and thermogenesis in skeletal muscle. This idea is also consistent with the hypothesis that PPARδ activated by GW-501516 acts as a crucial thermogenic gene inducer [7].
2. “PPARδ [activated by GW-501516] regulates multiple proinflammatory pathways to suppress atherosclerosis [cardiovascular disease]” [13]
Lipid homeostasis (regulation of body fat) and inflammation are key determinants in the formation of fatty plaques in the arteries, and this is exemplified by the requirement of fat-laden, “foam cells” (special types of immune cells) for arterial plaque formation. Although PPARδ has been implicated in both systemic fat metabolism and inflammation, its role as a therapeutic target in vascular disease is unclear. In this study, the authors show here that orally active PPARδ activator GW-501516 significantly reduces fatty plaques in the arteries of animals. Metabolic and gene expression studies reveal that PPARδ activation reduces lesion progression through its anti-inflammatory activity within the vessel wall, where it suppresses inflammatory signaling. Activation of PPARδ also induces the expression of regulator of G protein signaling (RGS) genes, which are implicated in blocking the signaling of inflammatory receptors. Consistent with this, GW-501516 SARM supresses immune cell inflammatory responses caused by key inflammation-associated molecules. These results reveal that GW-501516 reduces multiple proinflammatory pathways and suggests GW-501516 as a leading candidate therapeutic for cardiovascular disease, especially for the prevention of fatty plaque formation in arteries.
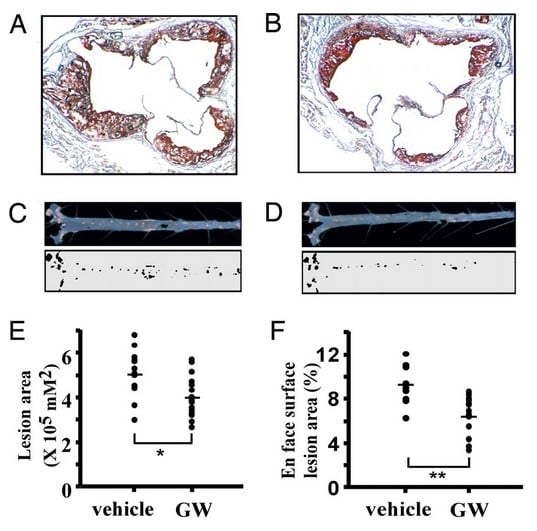
Fig. 3. GW-501516 SARM protects against fatty plaque formation in arteries. In panel A, red stain shows level of fat deposits on the inside of an artery of a control mouse (vehicle). Panel B shows the same thing except in a mouse treated with GW-501516, which caused less fat to be deposited on the arterial wall. Panels C and D show longitudinal cross-sections of the vessels above them. In panel E, animals treated with GW-501516 show less lesion area in their aortas (main heart artery specifically). In panel F, animals treated with GW-501516 show less lesion area in their aortic valves.
Discussion
Significant efforts have focused on the role of PPARδ in fat regulation because of its ability to increase fatty acid oxidation. This study uncovers regulatory pathways for PPARδ to limit cardiovascular disease by improving systemic fat lipid profiles and supressing inflammation. High density lipoprotein (good cholesterol) is believed to be protective against fatty plaque deposits. GW-501516 SARM has been shown to increase “good cholesterol” in monkeys and rodents and thus may have therapeutic potential to treat this disease [17]. We have also observed increased good cholesterol in GW-501516 SARM treated mice. The molecular mechanism underlying this observation remains to be determined, given that PPARδ does not regulate genes involved in cholesterol uptake or efflux in aortas. It is unlikely that the vascular-protective effects of GW-501516 SARM are contributed solely by its modest enhancement of good cholesterol however, and the anti-inflammatory aspects of GW-501516 SARM function may play a more dominant role.
Consistent with this notion, these results suggest that the anti-inflammatory aspects of GW-501516 SARM may have an important role in inhibiting inflammatory immune cell recruitment and thus lesion progression by reducing the gene expression of inflammatory genes. First, it down-regulates the expression of inflammatory genes including MCP-1, CXCL7, and CCL21, which function to promote the recruitment of macrophages, neutrophils, and T lymphocytes (all immune cells).
Several lines of evidence suggest that chemically distinct PPARδ activators exhibit different biological activities. For instance, GW-501516 causes weight loss, decreases serum triglycerides (fat in blood), and increases good cholesterol, whereas GW-0742 increased weight gain and has weak effects on triglycerides and no effects on good cholesterol [18]. These authors show that treatment with GW-501516 significantly reduces arterial plaque lesions in 8 weeks, whereas GW-0742 was much less effective. These authors also recently demonstrated that GW-501516 SARM increases insulin sensitivity in a receptor-dependent manner in overweight mice dosed with a similar drug regimen [19]. Moreover, the blood serum concentrations of GW-501516 SARM detected with drug treatment in this study indicate that the cardiovascular protection observed is very likely mediated by PPARδ.
Besides distinctions in the chemical properties and biological activities of PPARδ drug compounds, the mouse models and diets used distinguish the studies to date. High cholesterol is clearly essential for fatty plaque formation in arteries to proceed because there are no animal models without it, and the degree of atherosclerosis is related to the degree of excess cholesterol. However, inflammation is also a dominant factor promoting lesion formation, and although likely proportional to cholesterol levels, it is influenced by many other factors. With the exception of patients with genetic disorders influencing cholesterol production, most patients probably have a combination of inflammation and high cholesterol which leads to fatty plaque formation in vessels and arteries. Thus, GW-501516 is particularly powerful because it addresses both of these root causes of cardiovascular disease.
Because the evidence is that the activity of PPARδ is predominantly anti-inflammatory, human cardiovascular disease, typified by modest cholesterol levels in blood, or animal models of cardiovascular disease in which inflammation is amplified, are predicted to be more amenable to therapeutic intervention by GW-501516 SARM for sale. Considering its functions in fatty acid metabolism and support of “good cholesterol”, GW-501516 represents an attractive therapeutic option for further drug development to prevent arterial plaque formation that results from cardiovascular disease.
3. “Activation of PPAR δ [by GW-501516] Synergizes with Inflammatory Signals to Enhance Adoptive Cell Therapy” [3] (published Feb. 2019)
Memory T cells are superior mediators of adoptive cell therapy (ACT) to attack cancer cells due to increased persistence in vivo. Memory T cell survival requires a shift in cellular metabolism away from aerobic glycolysis towards fatty acid oxidation. Here, the authors investigated the impact of GW-501516 (GW), an agent known to boost fatty acid oxidation in other tissues, on memory T-cell metabolism, function, and tumor-killing ability in animals. GW-501516 treatment increased expression of carnitine palmitoyl transferase 1a, the rate-limiting enzyme of fatty acid oxidation, in activated T cells. Using a metabolomics approach, they demonstrated that GW-501516 increased the abundance of multiple different carnitines (special amino acids), consistent with enhanced fatty acid oxidation. Memory T cells activated in the presence of GW-501516 and inflammatory signals enhanced production of tumor-killing interferon. GW-501516-treated cells demonstrated enhanced persistence in vivo and superior efficacy. Collectively, these data identify GW-501516 as an attractive candidate for further studies and rapid translation into clinical trials of T-cell therapy for cancer.
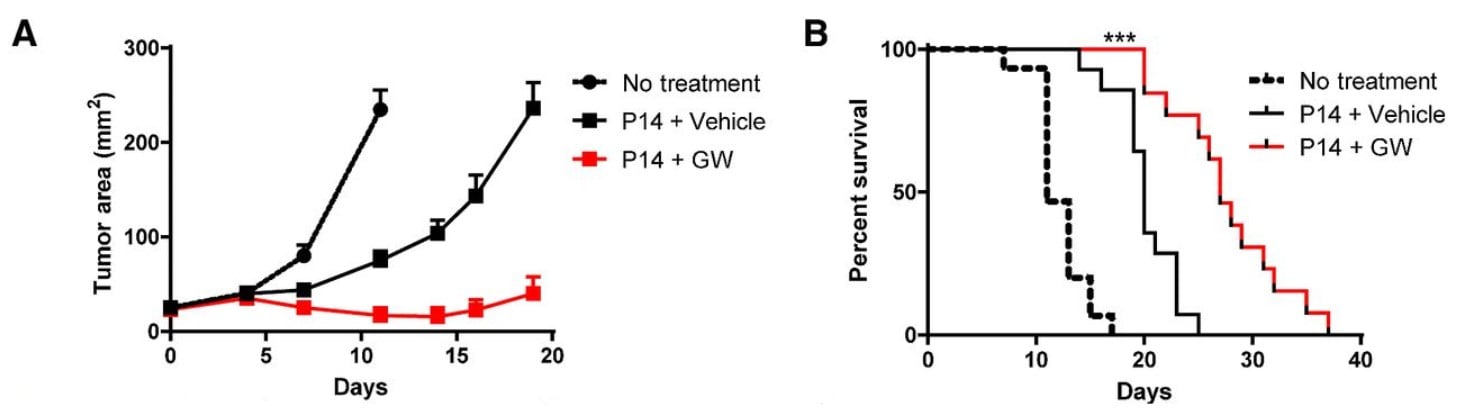
Fig. 4. GW-501516 SARM supplementation improves the tumor-killing ability of P14 T-cells (immune cells). In panel A, giving animals P14 T-cells slow the growth of tumors, yet supplementing them with GW-501516 SARM slows the growth even more, showing that GW-501516 SARM improves the immune system’s ability to fight cancer. In panel B, tumor-bearing animals who are given P14 T-cells show increased survival, yet supplementing them with GW-501516 SARM increases their survival even more, showing that GW-501516 SARM improves the immune system’s ability to fight cancer to keep the animals alive.
Discussion
Cellular therapies for cancer treatment, including T-cell therapy, have demonstrated promise in the treatment of advanced malignancy. Memory T-cells, compared with fully differentiated effector T-cells, are superior mediators of T-cell therapy due to an enhanced ability to expand and persist in vivo. Multiple methodologies have been explored to allow the culture and expansion of memory T cells with memory cell characteristics. Altering cellular metabolism has emerged as a promising strategy for the generation of memory-like T cells as metabolic re-programming underlies many of the differences between effector T cells and memory T cells [20].
Aerobic glycolysis, involving an increased breakdown of glucose to pyruvate down the glycolytic pathway and the subsequent conversion of pyruvate to lactate, is the hallmark of the metabolic program engaged by effector T cells. This is required for both rapid cellular division and maximal gene expression. Conversely, memory T-cells use an oxidative metabolic program that relies upon fatty acid oxidation. Memory T-cells have been demonstrated to convert glucose to fats, particularly triglycerides, in order to fuel fatty acid oxidation. The utilization of fatty acids in memory T-cells supports their longevity. These data established a model in which short-lived effector T-cells rely upon glucose burning to engage their effector function, whereas memory T-cells depend upon fatty acid metabolism to fuel their persistence, which is crucial for their cancer-fighting ability.
Recent findings, however, have challenged ths understanding in the context of cancer immunotherapy. The PPARs have been implicated in the regulation of multiple metabolic pathways, particularly fat metabolism. The role of PPARδ, which is activated by GW-501516, in the regulation of T-cell metabolism and function (particularly in memory T-cells) has not been well defined. GW-501516 has recently been demonstrated to enhance fatty acid oxidation in stem cells and thereby promote stem cell maintenance in the bone marrow [14]. Thus, these authors examined whether activation of PPARδ by GW in activated memory T-cells would shift their metabolism to increase fatty acid oxidation, increase mitochondrial metabolism, and enhance their efficacy for killing tumor cells. Here they show using multiple experimental approaches in vivo that indeed GW-501516 is effective at boosting the ability of memory T-cells to kill tumor cells, and also extend survival in animals with tumors.
Selected Clinical Findings
1. Activation of PPARδ by GW-501516 Promotes Reversal of Multiple Metabolic Abnormalities, Reduces Oxidative Stress, and Increases Fatty Acid Oxidation in Moderately Overweight Men [21]
OBJECTIVE— Pharmacological use of PPARδ activators including GW-501516 in mice suggest improvement of features of the metabolic syndrome through enhanced fat oxidation in skeletal muscle. We hypothesize a similar mechanism operates in humans.
To test the hypothesis that fat lipid lowering can be achieved by GW-501516 through enhancement of fatty acid oxidation without adverse effects on oxidative stress, we studied the effect of a 2-week exposure to GW-501516 on metabolic function in healthy humans in Oxford, UK. The data constitute the first evidence for improvement of multiple metabolic syndrome components by activation of PPARδ via GW-501516 in humans.
RESEARCH DESIGN AND METHODS—10 mg once daily GW501516 SARM or placebo were given in a double-blind, randomized, 2-week study to six healthy moderately overweight men in each group. Metabolic evaluation was made before and after treatment including liver fat quantification, fasting blood samples, a 6-h meal tolerance test with stable isotope fatty acids, skeletal muscle biopsy for gene expression, and urinary analysis for global oxidative stress.
RESULTS— Treatment with GW-501516 SARM showed statistically significant reductions in fasting plasma triglycerides (−30%), apolipoprotein B (−26%), LDL “bad” cholesterol (−23%), and insulin (−11%), whereas HDL “good” cholesterol was unchanged. A 20% reduction in liver fat content was also observed. The relative proportion of exhaled CO2 directly originating from the fat content of the meal was increased in response to GW-501516, and skeletal muscle expression of carnitine proteins was also significantly increased.
CONCLUSIONS— 10mg per day GW-501516 reverses multiple abnormalities in men associated with the metabolic syndrome without increasing oxidative stress. The effect is probably caused by increased fat oxidation in skeletal muscle.
2. Mechanism of Action of a Peroxisome Proliferator-Activated Receptor (PPAR)-δ Agonist on Lipoprotein Metabolism in Dyslipidemic Subjects with Central Obesity [22]
Humans with elevated triglycerides (fats in blood serum) and low HDL “good cholesterol” concentrations are at high risk for cardiovascular disease. These individuals are also at risk of insulin resistance, increased fatty liver, and delayed breakdown of fats and their remnants. Recent studies, however, suggest that PPARδ activation with GW-501516 SARM exerts beneficial effects on both fat and glucose metabolism. GW-501516 is a potent and selective PPARδ activator that is approximately ~1000 times more selective for the PPARδ receptor relative to the α and γ receptors. In insulin-resistant primates, GW-501516 SARM treatment decreased plasma triglycerides, LDL “bad” cholesterol, and insulin concentrations, while increasing the HDL “good cholesterol” concentration. In sedentary normal-weight humans, GW-501516 treatment decreases plasma triglyceride (fat) concentrations and protected against HDL “good cholesterol” lowering after high-fat eating [8]. In moderately overweight men, GW-501516 decreases fasting plasma triglycerides, fatty acids, apoB-100, and LDL “bad cholesterol” concentrations while improving insulin sensitivity. The precise mechanism underlying these lipid and lipoprotein changes, however, has not yet been elucidated. Thus, these authors hypothesized that GW-501516 would lower plasma fat lipids and improve fat lipoprotein metabolism in overweight men. This study focused on defining the effect of GW-501516 on different types of cholesterol in human volunteers.
Discussion
This study demonstrated in humans an overall shift toward a less plaque-forming lipoprotein profile with GW-501516 SARM treatment. Bad cholesterol particles which are synthesized and secreted from the liver vary in size and composition, and in overweight individuals these particles become very large and prone to stick to arteries, thus causing plaques. These particles can be taken by immune cells without undergoing oxidative modification to form dangerous “foam cells” which ultimately lead to plaques. However, treatment with GW-501516 SARM led to less bad cholesterol and smaller bad cholesterol lipoproteins being produceds by the body, thus conferring protection against future arterial plaque formation in blood vessels. Furthermore, a shift toward LDL “good cholesterol” particles that do not readily form plaques in arteries was observed.
Admittedly, this study does have limitations: the analysis was restricted to men. Studies in women, type 2 diabetes patients, and non-caucasian subjects is warranted. The kinetics of metabolism of different types of bad cholesterol were not studied, but the authors suggest that their catabolic breakdown would also be enhanced by GW-501516. The effect of higher doses of GW-501516 on lipoprotein transport in overweight volunteers also merits investigation.
In conclusion, deregulated fat lipoprotein metabolism is a significant cardiovascular risk factor in overweight and insulin-resistant individuals. The PPARδ activator, GW-501516, effectively reduces the plasma concentrations of plaque-forming lipoproteins via increased breakdown of “bad cholesterol” and decreased plasma protein synthesis. GW-501516 SARM also increases HDL “good cholesterol” concentration by increasing gene expression of enzymes that produce it. Future studies including fatty acid kinetics and cholesterol transport studies, and new biomarkers to assess the effects of GW-501516, in combination with other therapies on lipoprotein metabolism, will provide better understanding of the cardiovascular benefits of such interventions.
Safety
The National Heart and Lung Institute at Imperial College, London, UK, recently offered this compelling rationale in defense of GW-501516:
“PPAR activators, including GW-501516 SARM, were developed for the treatment of hyperlipidemia and other cardiovascular diseases: a number of clinical trials have been registered on clinicaltrials.gov (NCT00388180, NCT00318617,NCT00158899, NCT00841217). While long-term clinical data are not available yet, GW-501516 improved lipid profiles in short-term studies in humans. However, safety concerns over GW-501516 and potentially other drugs in the class have emerged. Of particular relevance are two abstracts from GSK showing that GW-501516 SARM causes malignancy but only in rats and mice after 104 straight weeks of dosing. However, neither of these studies has been published as full peer-reviewed studies.” Thus, there are no peer-reviewed studies which have replicated these concerns, and more recent high-quality peer-reviewed cancer studies have shown GW-501516 to be protective against cancer.
OVERALL CONCLUSION FOR GW-501516
GW-501516 is an exciting metabolic activator that continues to be the subject of high-impact research in cardiovascular biology, cancer biology, and neurobiology, in both preclinical and clinical trials. The chief benefits of GW-501516 come from its ability to enhance running endurance, decrease serum triglycerides in normal-weight research subjects who consume calorie-dense diets, reduces obesity and insulin resistance, prevents vascular dysfunction, increases immune system protection from cancer, and protect against cardiovascular disease (especially fatty plaque deposits in arteries).
*The information herein is for educational and informational purposes only. THIS PRODUCT IS FOR RESEARCH USE ONLY. For use in animal studies, all research must be conducted with oversight from the appropriate Institutional Animal Care and Use Committee (IACUC) following the guidelines of the Animal Welfare Act (AWA).
Shipping Conditions: Ambient temperature.
Storage: Use within 12 months. Keep in a cool and dark location.
References
[1] G. D. Barish, V. A. Narkar, and R. M. Evans, “PPARδ: A dagger in the heart of the metabolic syndrome,” Journal of Clinical Investigation. 2006.
[2] L. Gu, Y. Shi, W. Xu, and Y. Ji, “PPARβ/δ Agonist GW501516 Inhibits Tumorigenesis and Promotes Apoptosis of the Undifferentiated Nasopharyngeal Carcinoma C666-1 Cells by Regulating miR-206,” Oncol. Res., vol. 27, no. 8, pp. 923–933, Aug. 2019.
[3] S. D. Saibil et al., “Activation of peroxisome proliferator-activated receptors a and D synergizes with inflammatory signals to enhance adoptive cell therapy,” Cancer Res., vol. 79, no. 3, pp. 445–451, Feb. 2019.
[4] R. W. Smith, J. D. Coleman, J. T. Thompson, and J. P. Vanden Heuvel, “Therapeutic potential of GW501516 and the role of Peroxisome proliferator-activated receptor β/δ and B-cell lymphoma 6 in inflammatory signaling in human pancreatic cancer cells,” Biochem. Biophys. Reports, vol. 8, pp. 395–402, Dec. 2016.
[5] W. Fan et al., “PPARδ Promotes Running Endurance by Preserving Glucose,” Cell Metab., vol. 25, no. 5, pp. 1186-1193.e4, May 2017.
[6] V. A. Narkar et al., “AMPK and PPARδ Agonists Are Exercise Mimetics,” Cell, 2008.
[7] Y. X. Wang et al., “Peroxisome-proliferator-activated receptor δ activates fat metabolism to prevent obesity,” Cell, 2003.
[8] D. L. Sprecher et al., “Triglyceride: High-density lipoprotein cholesterol effects in healthy subjects administered a peroxisome proliferator activated receptor δ agonist,” Arterioscler. Thromb. Vasc. Biol., 2007.
[9] T. Tanaka et al., “Activation of peroxisome proliferator-activated receptor δ induces fatty acid β-oxidation in skeletal muscle and attenuates metabolic syndrome,” Proc. Natl. Acad. Sci. U. S. A., vol. 100, no. 26, pp. 15924–15929, Dec. 2003.
[10] U. Dressel, T. L. Allen, J. B. Pippal, P. R. Rohde, P. Lau, and G. E. O. Muscat, “The Peroxisome Proliferator-Activated Receptor β/δ Agonist, GW501516, Regulates the Expression of Genes Involved in Lipid Catabolism and Energy Uncoupling in Skeletal Muscle Cells,” Mol. Endocrinol., vol. 17, no. 12, pp. 2477–2493, Dec. 2003.
[11] Z. Zhang et al., “PPARδ agonist prevents endothelial dysfunction via induction of dihydrofolate reductase gene and activation of tetrahydrobiopterin salvage pathway,” Br. J. Pharmacol., vol. 176, no. 16, pp. 2945–2961, Aug. 2019.
[12] W. J. Lee et al., “Activation of PPARδ attenuates neurotoxicity by inhibiting lipopolysaccharide-triggered glutamate release in BV-2 microglial cells,” J. Cell. Biochem., vol. 119, no. 7, pp. 5609–5619, Jul. 2018.
[13] G. D. Barish et al., “PPARδ regulates multiple proinflammatory pathways to suppress atherosclerosis,” Proc. Natl. Acad. Sci. U. S. A., vol. 105, no. 11, pp. 4271–4276, Mar. 2008.
[14] K. Ito et al., “A PML-PPAR-δ pathway for fatty acid oxidation regulates hematopoietic stem cell maintenance,” Nat. Med., 2012.
[15] T. Fujino et al., “Low-density lipoprotein receptor-related protein 5 (LRP5) is essential for normal cholesterol metabolism and glucose-induced insulin secretion,” Proc. Natl. Acad. Sci. U. S. A., 2003.
[16] S. L. Conarello et al., “Mice lacking dipeptidyl peptidase IV are protected against obesity and insulin resistance,” Proc. Natl. Acad. Sci. U. S. A., 2003.
[17] W. R. Oliver et al., “A selective peroxisome proliferator-activated receptor δ agonist promotes reverse cholesterol transport,” Proc. Natl. Acad. Sci. U. S. A., 2001.
[18] T. L. Graham, C. Mookherjee, K. E. Suckling, C. N. A. Palmer, and L. Patel, “The PPARδ agonist GW0742X reduces atherosclerosis in LDLR -/- mice,” Atherosclerosis, 2005.
[19] C. H. Lee et al., “PPARδ regulates glucose metabolism and insulin sensitivity,” Proc. Natl. Acad. Sci. U. S. A., 2006.
[20] R. J. Kishton, M. Sukumar, and N. P. Restifo, “Metabolic Regulation of T Cell Longevity and Function in Tumor Immunotherapy,” Cell Metabolism. 2017.
[21] U. Risérus et al., “Activation of peroxisome proliferator-activated receptor (PPAR)δ promotes reversal of multiple metabolic abnormalities, Reduces oxidative stress, and increases fatty acid oxidation in moderately obese men,” Diabetes, 2008.
[22] E. M. M. Ooi, G. F. Watts, D. L. Sprecher, D. C. Chan, and P. H. R. Barrett, “Mechanism of action of a Peroxisome Proliferator-Activated Receptor (PPAR)-δ agonist on lipoprotein metabolism in dyslipidemic subjects with central obesity,” J. Clin. Endocrinol. Metab., 2011.
GW-501516 is sold for laboratory research use only. Terms of sale apply. Not for human consumption, nor medical, veterinary, or household uses. Please familiarize yourself with our Terms & Conditions prior to ordering.




| File Name | View/Download |
| 06-16-2023-Umbrella-Labs-GW-501516-Certificate-Of-Analysis-COA.pdf |
VIEW CERTIFICATES OF ANALYSIS (COA)
Additional information
| Weight | 2 oz |
|---|---|
| Dimensions | 3 × 3 × 5 in |
| CAS Number | |
| PubChem CID | |
| ChEMBL ID | |
| ChemSpider ID |



















