S-23 SARM POWDER – 1000MG / 1 GRAM
$70.99
S-23 SARM is sold for laboratory research use only. Terms of sale apply. Not for human consumption, nor medical, veterinary, or household uses. Please familiarize yourself with our Terms & Conditions prior to ordering.
*Includes:
- One (~10mg – 15mg) Red Micro Scoop
Also Available In:
![]() Liquid Option >>
Liquid Option >>
![]() Gel Option >>
Gel Option >>
- Description
- Additional information
Description
S-23 SARM Powder
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
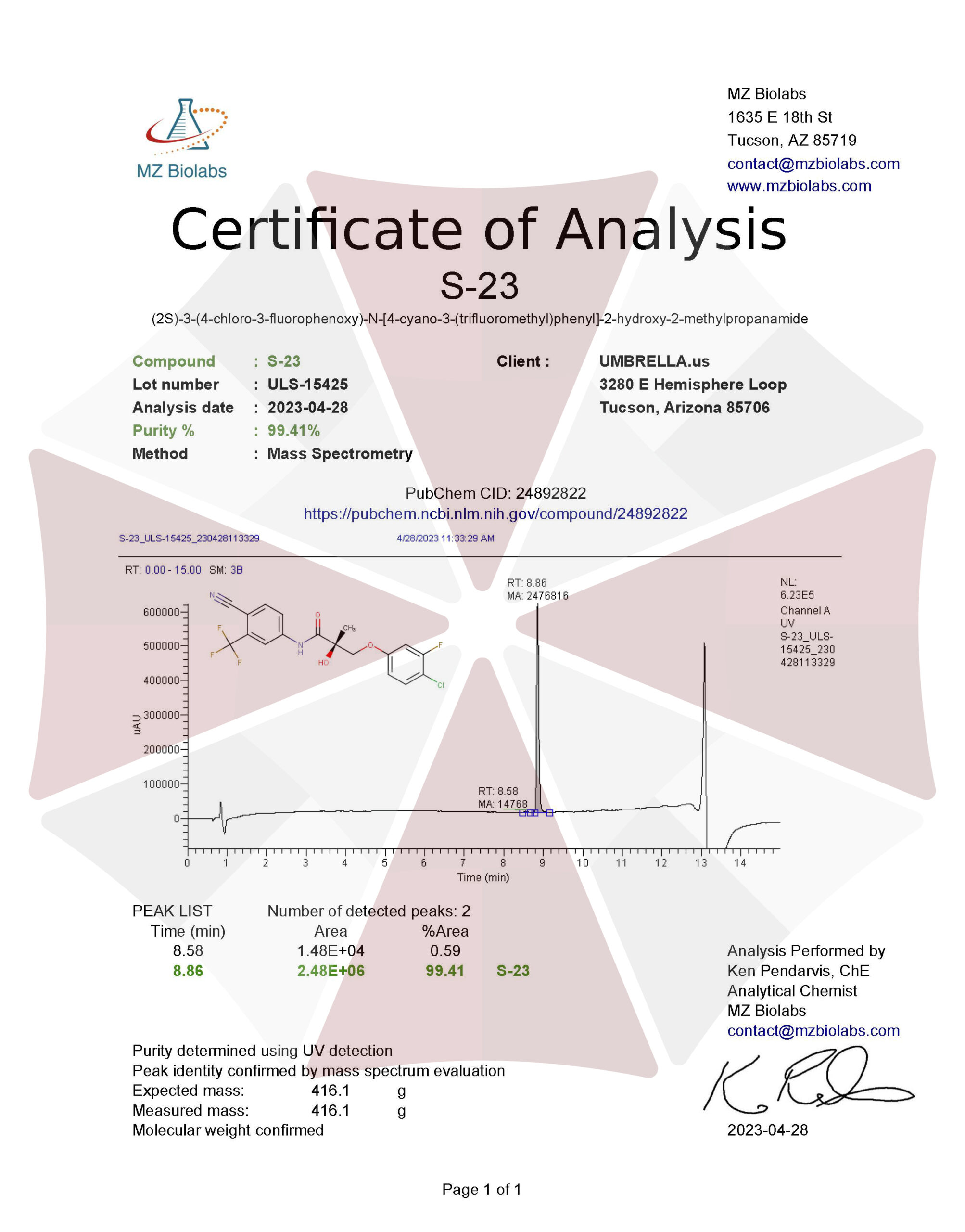
| CAS Number | 1010396-29-8 |
| Other Names | S23, S 23, CCTH-methylpropionamide, UNII-XDK89456WM, SCHEMBL2816704, SSFVOEAXHZGTRJ-UHFFFAOYSA-N, BCP30652 |
| IUPAC Name | 3-(4-chloro-3-fluorophenoxy)-N-[4-cyano-3-(trifluoromethyl)phenyl]-2-hydroxy-2-methylpropanamide |
| Molecular Formula | C₁₈H₁₃ClF₄N₂O₃ |
| Molecular Weight | 416.8 |
| Purity | ≥99% Pure (LC-MS) |
| Liquid Availability | |
| Powder Availability | |
| Gel Availability | |
| Storage | Store in cool dry environment, away from direct sunlight. |
| Terms | All products are for laboratory developmental research USE ONLY. Products are not for human consumption. |
*Includes:
- One (~10mg – 15mg) Red Micro Scoop
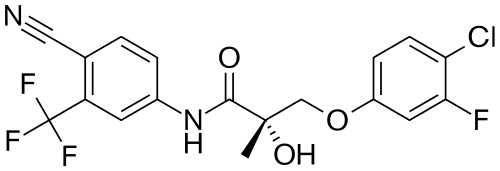
What is S-23?
The selective androgen receptor modulator (SARM), (S)-N-(4-cyano-3-trifluoromethyl-phenyl)-3-(3-fluoro,4-chlorophenoxy)-2-hydroxy-2-methyl-propanamide, is also commonly referred to as S-23. The compound has been shown to increase female libido, promote anabolic activity in muscles and bone tissue, and act as a male contraceptive as well as a partial agonist to the androgen receptor.
Main Research Findings
1) S-23 has the potential to act as a male contraceptive due to its overall beneficial effects and favorable pharmacokinetic profile.
2) In cases of glucocorticoid and hypogonadism-induced muscle atrophy, S-23 has been shown to enhance anabolic effects.
Selected Data
1) The initial portion of the study conducted by Jones et. Al assessed and confirmed the chemical purity of S-23 through the use of elemental analysis, mass spectrometry, and proton nuclear magnetic resonance. The first in vitro examination observed the transcriptional activation and AR binding affinity of the SARM through the use of a radiolabeled competitive binding assay and a cotransfection assay. 24 hours after castration took place cytosolic AR was obtained from the ventral prostate of male Sprauge-Dawley rats. AR-mediated transcription elicited by the agonist qualities of S-23 were compared to the control, defined in this study as the AR activation following treatment with 1 nM DHT [2].
The first in vivo experiment attempted to observe patterns in anabolic and androgenic activity following treatment with S-23 in castrated male rats. 30 male Sprague-Dawley rats weighing approximately 200 g were castrated via scrotal incision 24 hours prior to administration of the first drug treatment. 0.01, 0.05, 0;1, 0.5, 1, and 3 mg doses of S-23 were subcutaneously injected daily, over a treatment period of 2 weeks. Two additional groups were introduced, one castrated one intact, as control groups after receiving doses of a vehicle treatment. Animals were euthanized 24 hours after treatment; plasma samples were collected while the ventral prostate, seminal vesicles, and levator ani muscle were extracted and weighed. Androgenic activity was evaluated by weight changes in the prostate and seminal vesicles, and anabolic activity was measured by weight changes in the levator ani. In order to determine the maximal response (Emax) induced by S-23 the research team used nonlinear regression analysis [2].
The next portion of the study determined whether or not S-23 has the potential to act as a male contraceptive by observing the effects the SARM has on spermatogenesis and endocrine physiology in intact male rats. 42, 90-day-old male Sprauge-Dawley rats were assigned to seven different groups and subcutaneously administered different drug treatments. Group 1 was the intact control group that received only a vehicle composed of 5% DMSO and 95% PEG300. Group 2 was a control group that was hormone-primed with 5 ug/day of estradiol benzoate (EB). In addition to 5 ug/day of EB, groups 3-7 received doses of S-23 varying from 0.05, 0.1, 0.3, 0.5, and 0.75 mg/day, respectively.
The compounds were prepared accordingly and administered to the animals daily for 70 days. S-23 has high oral bioavailability, however, the research team used a subcutaneous method to reduce stress and morbidity in the animals while undergoing long-term treatment. Mating trials were conducted the last week of treatment, specifically in groups 1, 2, 4, and 5, to ensure the animals in each group were demonstrating proper mounting and copulatory behavior. Individual male rats from each group were cohabited with two sexually active females while the research team recorded the number of mounts, mount latency, intromissions, and intromission latency. After the female rats were impregnated they were allowed to carry to full term. The number of successful pregnancies was reported and male rats were only defined as fertile if he impregnated one or two female partners [2].
A secondary study was conducted with separate experimental groups where the animals were left untreated for 70 days following 70 days of drug treatment. On day 140 a second mating trial was performed and on day 168 a third mating trial was performed for the subjects that were deemed infertile on day 140. Whole body DEXA scans were taken one day before the end of treatment in order to record body weight, bone mineral density (BMD), percent fat mass (FM), and fat-free mass.
At the end of the treatment period the rats were anesthetized and euthanized. Body weight was recorded again at autopsy while the prostate, seminal vesicles, right testis, and right epididymis were extracted and weight. Blood samples were collected and maintained at room temperature followed by centrifugation for 10 minutes and preparation for further analysis. One testis was removed from each subject and weighed. Testicular parenchyma was then homogenized in order for each testicular homogenate to be used to count advanced spermatids. Remaining homogenates were centrifuged so the supernatant of each sample could be collected and used to quantify intratesticular testosterone concentrations. Statistical analysis was performed using single-factor ANOVA and Dunnett’s multiple comparison test [2].
2) The research team of Jones et. Al examined the potential of S-23 to treat muscle atrophy induced hypogonadism as well as chronic glucocorticoid usage, in this case, dexamethasone (DEX). Male Sprague-Dawley rats aged 5-6 weeks old and weighing 229-238 g were obtained from Harlan Bioproducts for Science. Food and water was available ad libitum and the animals were kept on a strict 12-hour dark, 12-hour light cycle. Before and after treatment the research team measured body fat, lean body mass (LBM), fluids, and total water via MRI screening. The rats were then randomized according to LBM and assigned to one of four groups. Animals were subcutaneously injected with a treatment dose of the compound of interest over an experimental period of 8 days. Group 1 was defined as the control group and was administered only a vehicle while groups 2-4 were administered 600 ug/kg of DEX; groups 3 and 4 received the same dose of DEX as well as 25 mg/kg of testosterone propionate (TP), or S-23 [1].
24 hours after the last dose the animals were weighed and euthanized. The ventral prostate and seminal vesicles were extracted and weighted while the levator ani, gastrocnemius, extensor digitorum longus (EDL), and soleus muscle were extracted, weight and segmentally preserved in RNAlater for gene expression and protein expression analysis. Serum testosterone was analyzed through the use of liquid chromatography and tandem mass spectrometry. Cell cultures were taken from the myoblasts of the mouse skeletal muscle cell line and cultured in a growth medium composed of DMEM, 10% FBS, 100 U/ml penicillin, and 100 ug/ml streptomycin.
Additionally, quantitative real-time PCR was performed by seeding mouse skeletal muscle cells into six-well plates in medium at a density of 1 million cells/wells, followed by an 8 hour recovery period. After 24 hours cells were transduced with 250 virus particles/well and incubated for another 24 hours. After incubation cells were treated with the compound of interest and incubated for another 24 hours before muscle sample analysis took place. RT PCR was followed by Western blotting analysis. The mouse skeletal muscle cells were seeded into 10 cm dishes in a medium at a density of 2.5 million cells/dish. Cells were transduced and treated in the same manner as the RT PCR, and were washed with PBS before the protein was extracted in a buffer solution and subjected to further analysis. Finally, statistical analysis was performed through the use of single-factor ANOVA and Dunnet’s multiple comparison test [1].
An additional group of male Sprague-Dawley rats was obtained from Harlan Bioproducts in order to conduct a time-course study. 24 hours before drug treatment the animals were orchidectomized via scrotal incision. S-23 was then administered subcutaneously at a dose rate of 1 mg per day. Intact and castrated muscles were administered a vehicle treatment and used as the control groups; all animals were treated for 3, 7, 19, 14, 21, or 28 days. 24 hours after the last treatment the animals were weighed and euthanized while the ventral prostate, seminal vesicles, levator ani, gastrocnemius, EDL, and soleus muscles were extracted, weighed, and preserved for further gene expression analysis. These animals were subjected to the same cell culturing, RT PCR, Western blotting, and statistical analyses, as the primary experimental group [1].
Discussion
1) The in vitro portion of the study conducted by Jones et. Al reported that S-23 led to a significant increase in transcription activation mediated by the androgen receptors when compared to the transcriptional activity elicited by 1 nM of DHT. The in vivo pharmacological portion of the study found that the weight of the prostate, seminal vesicles, and levator ani decreased after the animals were castrated. Additionally, androgen-dependent organs experienced a dose-dependent increase in weight when S-23 was administered to the castrated animals. When the animals were given 1.0 mg/day of S-23 the weight of the prostate and seminal vesicles were maintained at weights equal to or greater than that of intact animals. Weight of the levator ani was selectively maintained in doses as low as 0.1 mg/day. The Emax of S-23 in the prostate, seminal vesicles, and levator ani muscle was found to be 138 ± 21, 144 ± 1, and 129 ± 4%, respectively [2].
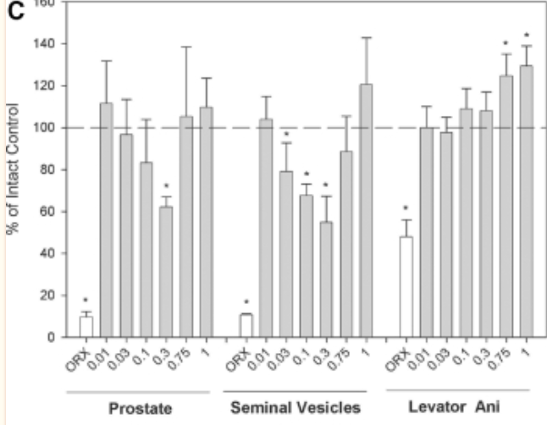
Figure 1: Androgenic and anabolic activity of S-23 in intact male rats
Mating trials took place at the end of the treatment period in order to assess the efficacy of S-23 as a male contraceptive. Estradiol benzoate was administered in addition with the SARM in order to ensure sufficiently restored mating behavior in the castrated male rats. Results reported that the weight of the levator ani muscle in castrated male rats was maintained at doses between 0.1 and 0.3 mg/day. Researchers observed groups 1, 2, 4, and 5: vehicle, EB only, EB + 0.1 mg S-23, and EB + 0.3 mg S-23, respectively. There were no observed differences in the number of mounts, mount latency, number of intromissions, or intromission latency between the four groups, allowing the research team to conclude that EB alone and EB + S-23 were able to maintain normal sexual behavior relative to the control group.
Contraception efficacy was measured by the fertility rate of the male rat included in the mating trials. Results found that all male rats treated with EB + 0.1 mg of S-23 were infertile while only one of six male rats treated with EB + 0.3 mg of S-23 was fertile. In terms of spermatogenesis, it was first evaluated by counting the number of homogenization-resistant advanced spermatids from control and experimental rats. There were no differences in mean sperm count between the intact control animals and the EB-treated animals. When EB was administered with S-23, the SARM elicited a biphasic effect on sperm count in the testis. This response is directly related to the androgenic activity of drug treatment in the sexual organs. Spermatogenesis was significantly inhibited in the treatment group receiving EB + 0.1 mg/day of S-23. Four out of the six animals in the treatment group exhibited no sperm in the testis while sperm count was barely detectable in the other two animals [2].
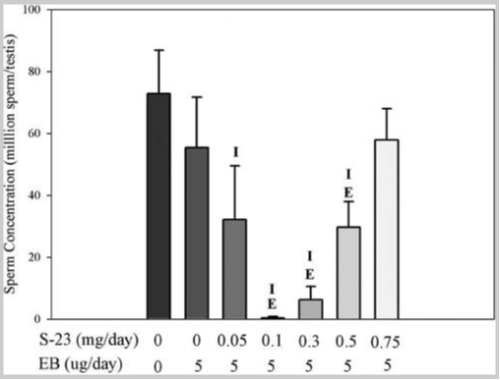
FIgure 2: Testicular sperm concentrations
After the test subjects were euthanized the body weight of each animal was recorded. All EB-treated groups exhibited significantly lower body weight and prostate weight, however, total body BMD was greater than that in the intact, vehicle-treated group. The animals that received S-23 experienced a decrease in the weight of the testis while weight of the levator ani muscle remained unchanged. Treatment with EB alone did not significantly change fat mass in comparison to the intact control group. When EB was administered with S-23 fat mass was shown to decrease in a dose-dependent manner; doses of 0.3 mg/day of S-23 and higher, led to a significant diminishment of fat mass in comparison to the intact control group. Overall, the researchers were able to conclude that S-23 treatment in addition to EB administration, effectively decreases fat mass [2].
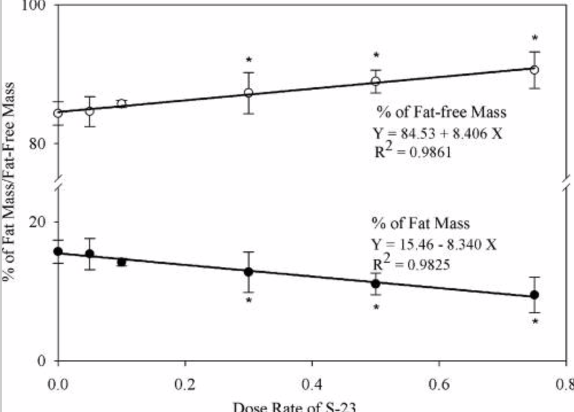
Figure 2: dose-dependent changes in fat mass elicited by S-23
2) First, the research team of Jones et. Al began the study by AR-transducing mouse skeletal muscle cells and incubating overnight with DEX in order to induce atrophy. DEX incubation led to a 6.5-fold increase in expression of MAFbx mRNA. When the samples were treated with TP or S-23, up-regulation of mRNA expression was significantly inhibited. DEX incubation also led to a 13-fold decrease in the expression of IGF-1 mRNA; this response was inhibited by TP and S-23 at concentrations greater than 1 nM. Adding DEX to the mouse skeletal muscle cells led to the reduction in phosphorylation of proteins involved in the PI3K/Akt pathways.
Intact male rats experienced a significant reduction in total body weight after administration of DEX. Cotreatment with TP and S-23 inhibited the overall effects of DEX on body weight. DEX treatment also led to a 5% reduction in LBM while TP only partially inhibited this affection. DEX also significantly reduced prostate and seminal vesicle size. When coadministering an androgen treatment, the weights of these aforementioned tissues significantly increased. The levator ani muscle was extracted in order to measure weight changes in response to DEX. Results reported that weight of the levator ani decreased by 52% when DEX was administered. This decrease in weight was inhibited by TP and S-23 administration; the weight of the muscle was similar to that in the intact control group. In response to DEX treatment the gastrocnemius muscle weight was reduced by 37%, while the EDL also reduced in size by 34%. It is important to note that when TP and S-23 were administered, no attenuation of the effects were observed. The soleus muscle also experienced a decrease in size following DEX administration, however, this response was inhibited by S-23 but not TP.
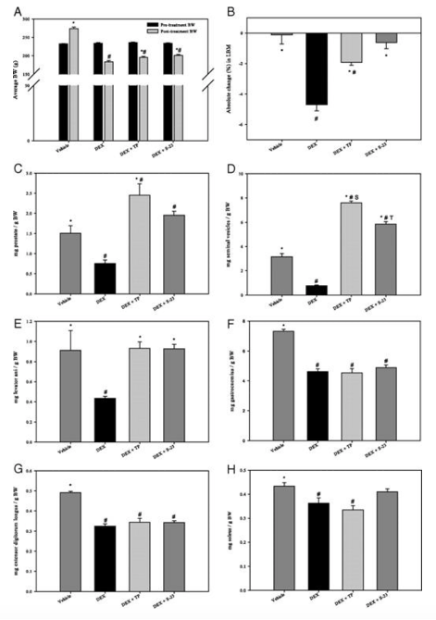
Figure 4: Effects of treatment on body weight, LBM, and androgenic/anabolic activity. A) average body weight, B) absolute change in LBM, C) normalized prostate weights, D) seminal vesicles, E) levator ani muscle, F) gastrocnemius muscle G) EDL, H) soleus muscle
When administering DEX to intact animals, the subjects experienced an upregulation in MAFbx mRNA in the levator ani, gastrocnemius, EDL, and soleus muscles. Coadministration of TP and S-23 inhibited upregulation of mRNA expression in the levator ani while expression in the gastrocnemius, EDL, and soleus was significantly inhibited by S-23 and only slightly by TP. A similar trend was observed with the expression of . TP and S-23 inhibited upregulation of mRNA expression in the levator ani, while only S-23 led to a significant inhibition in expression in the gastrocnemius, EDL, and soleus muscles. Levels of IGF-1 were decreased by DEX administration, specifically in the gastrocnemius, EDl, and soleus by 3-, 6.5, and 5.5-fold, respectively. The levator ani muscle did not experience any changes in IGF-1 expression. Again, S-23 was more effective than TP in inhibiting the expression of IGF-1 in the gastrocnemius, EDL, and the soleus muscle.
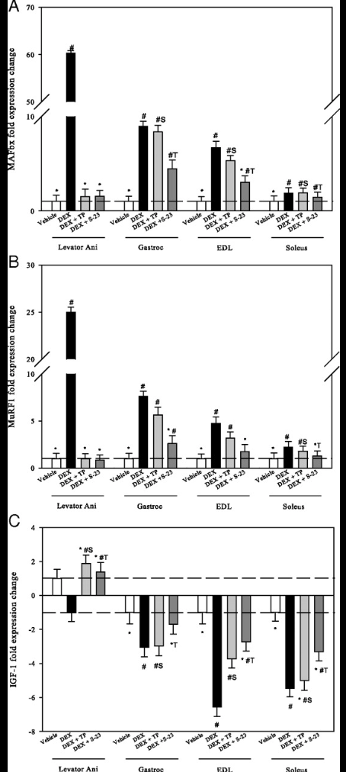
Figure 5: Effects of treatment on gene expression. A) MAFbx, B) MuRF1, C) IGF-1
In regards to the secondary experimental group examining the effects of time and treatment with S-23 in castrated rats. All subjects were castrated in order to induce hypogonadism-related muscle atrophy. 3 days post-orchiectomy, the prostate and levator ani sizes were reduced by 67 and 79% of the intact control group, respectively. Over the course of 14 days both tissues continued to decrease in weight. When S-23 was administered to the orchidectomized animals, the weight of both the prostate and the levator ani were maintained. The weight of each organ continued to increase in the animals treated with the SARM for 28 days. In terms of gene expression, castration led to a 40-fold increase in MAFbx mRNA expression at day 3, however, this increase levels off by day 14. S-23 inhibits the upregulation of mRNA expression at 3, 7, and 10 days, but no further changes were observed past day 14. Additionally, the researchers thought it was important to note that IGF-1 was down-regulated in response to castration but his response was inhibited by S-23.
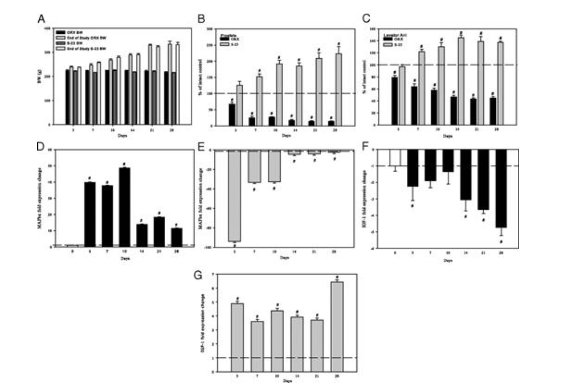
Figure 6: Effects of time and S-23 on androgenic and anabolic activity in the tissues of castrated male rats. A) Body weights before and after treatment, B) normalized prostate weight of castrated control and S-23 treated animals, C) normalized levator ani weight of castrated control and S-23 treated animals, D) expression change of muscle atrophy marker MAFbx in orchidectomized subjects, E) expression change of muscle atrophy marker MAFbx in orchidectomized subjects each day, F) Fold expression change of muscle hypertrophy marker IGF-I, in orchidectomized animals compared to the control, and G) Fold expression change of muscle hypertrophy marker IGF-I in S-23 treated animals compared to orchidectomized animals each day
Disclaimer
**LAB USE ONLY**
*This information is for educational purposes only and does not constitute medical advice. THE PRODUCTS DESCRIBED HEREIN ARE FOR RESEARCH USE ONLY. All clinical research must be conducted with oversight from the appropriate Institutional Review Board (IRB). All preclinical research must be conducted with oversight from the appropriate Institutional Animal Care and Use Committee (IACUC) following the guidelines of the Animal Welfare Act (AWA).
Citations
[1] Jones A, Chen J, Hwang DJ, Miller DD, Dalton JT. Preclinical characterization of a (S)-N-(4-cyano-3-trifluoromethyl-phenyl)-3-(3-fluoro, 4-chlorophenoxy)-2-hydroxy-2-methyl-propanamide: a selective androgen receptor modulator for hormonal male contraception. Endocrinology. 2009 Jan;150(1):385-95. doi: 10.1210/en.2008-0674. Epub 2008 Sep 4. PMID: 18772237; PMCID: PMC2630904.
[2] Jones A, Hwang DJ, Narayanan R, Miller DD, Dalton JT. Effects of a novel selective androgen receptor modulator on dexamethasone-induced and hypogonadism-induced muscle atrophy. Endocrinology. 2010 Aug;151(8):3706-19. doi: 10.1210/en.2010-0150. Epub 2010 Jun 9. PMID: 20534726.
S-23 SARM is sold for laboratory research use only. Terms of sale apply. Not for human consumption, nor medical, veterinary, or household uses. Please familiarize yourself with our Terms & Conditions prior to ordering.




| File Name | View/Download |
| 04-28-2023-Umbrella-Labs-S23-Certificate-Of-Aunalysis-COA.pdf |
VIEW CERTIFICATES OF ANALYSIS (COA)



















