OXIRACETAM POWDER (50 GRAMS)
$39.99
Oxiracetam is sold for laboratory research use only. Terms of sale apply. Not for human consumption, nor medical, veterinary, or household uses. Please familiarize yourself with our Terms & Conditions prior to ordering.
- Description
- Additional information
Description
Oxiracetam Nootropic Powder
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
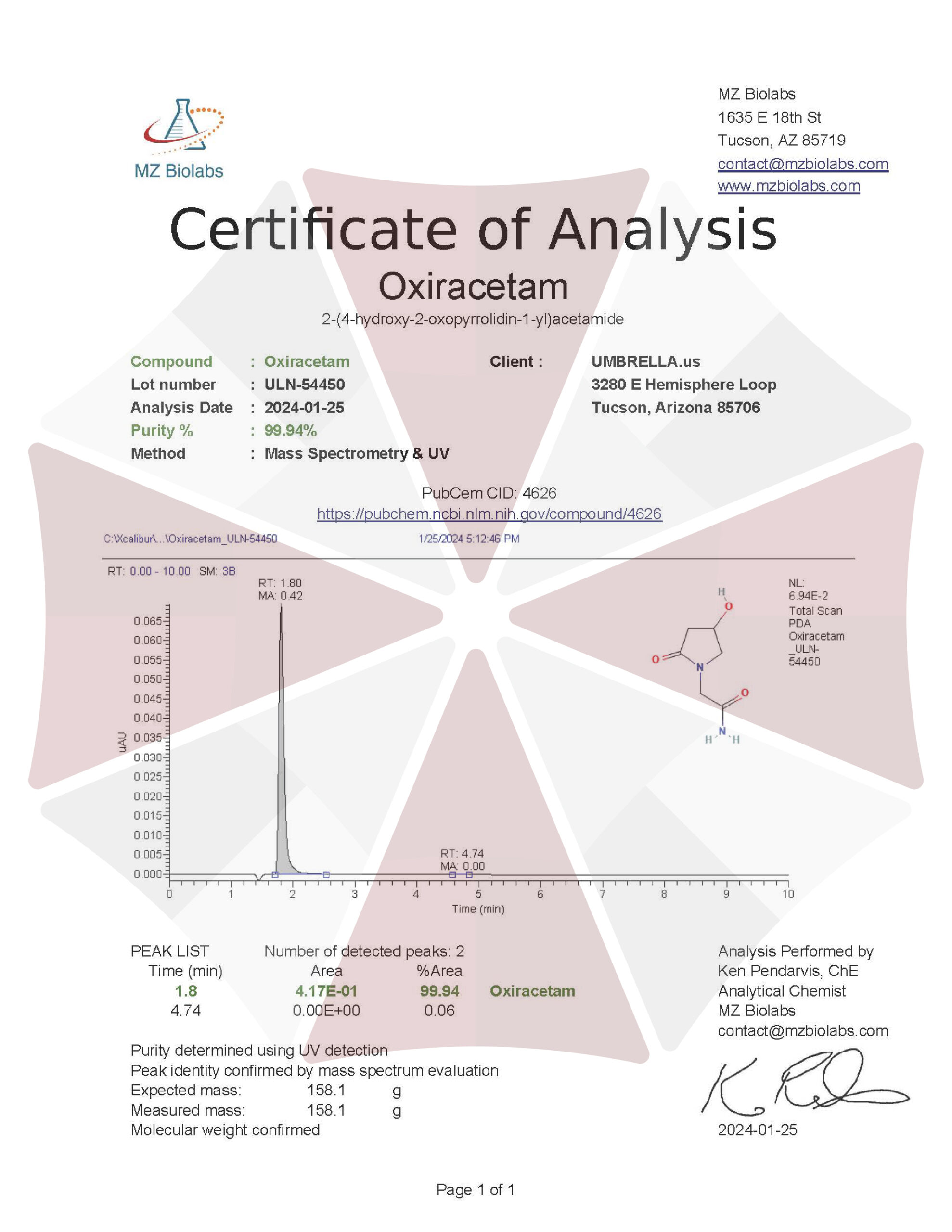
| CAS Number | 62613-82-5 |
| Other Names | ISF 2522, CGP 21690E, ISF-2522, CT-848, CGP-21690E, GNF-PF-1005 |
| IUPAC Name |
(RS)-2-(4-hydroxy-2-oxopyrrolidin-1-yl)acetamide
|
| Molecular Formula | C₆H₁₀N₂O₃ |
| Molecular Weight | 158.15 |
| Purity | ≥99% Pure (LC-MS) |
| Liquid Availability | N/A |
| Powder Availability | |
| Gel Availability | N/A |
| Storage | Store in cool dry environment, away from direct sunlight. |
| Terms | All products are for laboratory developmental research USE ONLY. Products are not for human consumption. |
What is Oxiracetam?
Oxiracetam (4-hydroxy-2-one-1 pyrrolidine acetamide) is a nootropic compound from the racetam family that is known for its ability to directly affect the hippocampus and cerebral cortex by passing through the blood-brain barrier. Oxiracetam is thought to be relatively safer and well-tolerated considering that the nootropic has no effect on vascular activity, nor does it exhibit any excitability of the central nervous system. That being said, current research regarding the compound focuses on its neuroprotective qualities that may be used to improve impaired cognitive function and the clinical outcomes of several CNS disorders and neurodegenerative diseases [2].
Main Research Findings
1) Administration of Oxiracetam was found to potentially assist in improving neuroinflammation and restoring cognitive impairments in cases of traumatic brain injury (TBI).
2) Treatment with Oxiracetam assists with the mediation of neuroprotective effects by regulating microglia activity in mice experiencing hypoxia-ischemia neonatal brain injury.
Selected Data
1) When traumatic brain injury occurs, cognitive impairments are frequently seen as a side effect and have been shown to reach 49% of patients in the subacute phase and 54% in the chronic phase. While the drug donepezil is sometimes prescribed for patients with significant cognitive impairments, there is no treatment for impairments following a TBI. That being said, current research has focused on the nootropic, Oxiracetam, for its potential to improve executive functioning and memory through the enhancement of hippocampal protein kinase C (PKC) and the enhanced activity of K+ induced acetylcholine and choline acetyltransferase. Researchers have been able to conclude that the neuroinflammation that occurs post-TBI directly impacts cognitive function. That being said, the research team of Youn et al observed the potential abilities of Oxiracetam to alleviate excessive neuroinflammation to recover normal cognitive functioning [1].
Both an in vitro and in vivo models to demonstrate the effects of Oxiracetam on cognitive functioning following TBI. using Ham’s F12/Minimum Essential Medium, SH-SY5Y cells were introduced and grown to 80-90% confluence in six-well BioFlex culture plates. Once 80-90% confluence was achieved, the cells were then incubated in a 1:1 ratio of Ham’s F12/Minimum Essential Medium. For the purpose of model induction, the researcher used a Cell Injury Controller II system with 50 ms pulse duration and a regulator pressure of 3.5 ms.
All cells were then randomly divided into three groups: control, TBI with no treatment, and TBI treated with 100 mM of Oxiracetam; this dose was determined through the use of the CCK-8 assay. Following the culturing period, the cells were then subjected to quantitative real-time polymerase chain reaction (qRT- PCR) analysis to assess the mRNA expression of SOD1, SOD3, TNF-alpha, IL-1, IL-10, and IL-1beta. qRT-PCR was followed by the performance of Western blotting analysis in order to measure the expression of COD-2, NACHT, LRR and NLRP3, caspase-1, and IL-1beta. Finally, DCF-DA staining and terminal deoxynucleotidyl transferase dUTP nick end-labeling too place to examine the production of intracellular reactive oxygen species and the level of apoptotic cells present [1].
For the in vivo portion of the study, the research team used seven- or eight-week old male C57BL/6 J mice. The animals were maintained under standard conditions including a 12-hour dark/light cycle, and food and water provided ad libitum. Similar to the in vitro portion of the study, the mice were randomly divided into three groups including: sham operation, TBI with no treatment, and TBI with Oxiracetam treatment. TBI was induced in the mice through the use of a stereotaxic impactor, followed by placing the mice in a stereotaxic frame under 2.5% isoflurane anesthesia. The scalps were exposed and TBI was generated using a 2 mm blunt tip placed at a depth of 2.0 mm, -2.0 mm in the medial/lateral direction, and -2.0 mm in the anterior/posterior direction. [1]
Following the induction of TBI or sham surgery, Oxiracetam was administered intraperitoneally to the experimental group in doses of 30 mg/kg/day over a treatment period of 5 days. In order to reduce the risk of bias throughout the study, only investigators that were blinded to the treatment protocol evaluated the outcomes. In order to observe the effects of the nootropic in test subjects involved in the in vitro study, three different cognition tests including: novel object recognition, the Y-maze, and the Morris water maze, were performed. The tests were recorded using a video tracking system and analyzed using heat mapping with red representing frequently visited areas and blue indicating less-visited areas [1].
2) Hypoxic and ischemic conditions in the brain lead to cellular necrosis and tissue liquefaction, ultimately leading to inflammation that further increases the deterioration of brain tissue and expansion of the infarct core. If treatment is not administered in a timely manner, the area of recoverable tissue that surrounds the infarct core is prone to expansion, resulting in secondary impairments and potential incorporation into the core, leading to expansion of the infarction. That being said, the research team of Wang et al examined the effect of treatment with Oxiracetam on the recovery from neural damage caused by hypoxia, ischemia, and the following inflammatory cascade, as well as microglia activation in the brain region.
The research team utilized male KM mice that were 7 days postnatal for their experimental purposes. The test subjects were randomly assigned to 4 experimental groups including: 1) a sham group treated with physiological saline, 2) mice that underwent hypoxia-ischemia insult, 3) mice that underwent the insult and were treated with 1.0 mg/kg of Oxiracetam, and 4) mice that underwent the insult and were treated with 10.0 mg/kg of Oxiracetam. The mice were anesthetized through the inhalation of isoflurane gas while a dissecting microscope was used to make a permanent ligation of the right carotid artery [2].
After the procedure the test subjects were placed in a recovery chamber for 60 minutes followed by exposure to hypoxic conditions (humidified 8% O2 + 5% CO2 + 87% N2) for 90 minutes; the mice in the control group underwent anesthesia and the neck incision only. The first dose of Oxiracetam of saline was injected intraperitoneally, 6 hours after the hypoxic-ischemic insult. Dosage administration continued for 3 days, followed by euthanasia of the mice for further analysis of the brain tissue.
Prior to the animals being euthanized, three different behavioral reflex tests were performed on the mice including the righting reflex test, the negative geotaxis reflex test, and the cliff avoidance reflex test. The righting reflex test includes having the mice supine on a horizontal table top while the researchers record how long it takes for each group of mice to land on all four limbs after being turned over. The maximum time allowed was 1 minute and each mouse was tested 3 times. The negative geotaxis reflex test includes placing each mouse in the middle of a rough, 30-cm board at a 25 degree incline with their head pointing downward while the researchers recorded the time it took for each group of mice to turn 180 degrees and climb down the board. The maximum amount of time allowed was 3 minutes and each mouse was tested 3 times. Finally, the cliff avoidance reflex test included placing the mice on the edge of a platform with their forepaws and nose extending over the edge while the researchers recorded the time it took for each group of mice to leave the table away from 1 cm [2].
After the behavioral reflex testing was performed, MRI scans were completed in order to identify the extent of the infarction; the animals were anesthetized through isoflurane inhalation and scanned for 0.5 hours. Once the animals completed the MRI they were euthanized and the brain tissue was collected and brain water content calculation was performed through the dry-wet weight method. The infarct ratio was measured next through TTC infarct measurement techniques. The brain tissues were sectioned into 1 mm thick slices and incubated in 2% TTC for 10 minutes, followed by fixation of the slices in 4% paraformaldehyde for 24 hours [2].
In order to assess the activation of microglia in the area of salvageable damage, primary microglia cell cultures were prepared by digesting samples of the cerebral cortex in 0.25% trypsin EDTA for 20 minutes, followed by the termination of digestion with DMEM containing 10% fetal bovine serum, penicillin, and streptomycin. The resulting product was filtered, resuspended in medium, and seeded in flasks that were maintained for 8-10 days prior to separating the microglia cells. The sample cells were seeded onto 6-well plates for 2-3 days and were then harvested for the experimental oxygen-glucose deprivation model.
Both the primary microglia cells and HT-22 cells were utilized for the oxygen-glucose deprivation model and initially washed with PBS followed by incubation in a low-glucose medium. All cells were then randomly assigned to one of five experimental groups including: 1) control, 2) oxygen-glucose deprivation, 3) oxygen-glucose deprivation with administration of 50 nmol/ml of Oxiracetam, 4) oxygen-glucose deprivation with administration of 100 nmol/ml of Oxiracetam, and 5) oxygen-glucose deprivation with administration of 200 nmol/ml of Oxiracetam. The HT-22 cells and primary microglia cells were further divided into 5 time point subgroups including: 0 hours, 3 hours, 6 hours, 9 hours, and 12 hours). The cells were then seeded into culture plates and incubated in order for cell proliferation to be analyzed through the use of CCK-8 assay to observe how treatment with Oxiracetam affects cells incubated in a low glucose medium. Additionally, the RNA of the microglia cells treated with the low glucose medium with or without treatment with the nootropic, were extracted and prepared for RNA quality examination [2].
Discussion
1) The results of the CCK-8 assay utilized the in vivo portion of the experiment conducted by the research team of Youn et al, revealed that there was no significant difference in the viability of the cells treated with less than 100 nM of the nootropic, Oxiracetam. That being said, doses of Oxiracetam of 100 nM and above found that the nootropic caused higher expressions of SOD1 and SOD2, while reducing the levels of TNF-alpha, IL-6, IL-10, COX-2, NLRP3, caspase-1 and IL-1 beta mRNA and proteins. Additionally, the nootropic lowered the number of TUNEL-positive cells and DCF-DA fluorescent cells [1].

Figure 1: Changes in (A) cell viability, (B) SOD1, (C) SOD2, (D) TNF-alpha, (E) IL-6, (F) IL-10, (G) COX-2, (H) NLRP3, (I) Caspase-1, and (J) IL-1 beta
For the in vivo portion of the study, the test subjects were split into 3 groups of 20 mice each representing a control group that underwent a sham operation, a group that had TBI induced, and an experimental group that had TBI induced and treated with Oxiracetam. Several samples were obtained from the mouse and procedures were followed in order to assess the effects of the nootropic on RNA and protein, IF staining, BWC, and TTC staining. Results gathered from these samples and additional imaging revealed that the injured brains experienced a significant loss of cortical tissues following the induction of TBI. However, in the subjects treated with Oxiractam there was a marked reduction in the volume of cortical tissue lost following TBI.
In addition to a reduction in cortical tissue loss, Oxiracetam was also able to decrease ipsilateral cortical volume loss and cerebral edema when compared to the other groups of test subjects. These findings were reported through TTC staining of brain slices. Administration of the nootropic also decreased the number of FJB-positive and TUNEL-positive cells, as well as levels of COX-2 NLRP3, caspase-1, and IL-1 beta mRNA and protein expression. Out of all of the levels tested, NLRP3 exhibited the greatest difference between the test subjects with induced TBI that did or did not receive an administration of the nootropic [1].


Figure 2: Changes in (C) Brain water content and area of brain tissue loss, (E) COX-2, (F) NLRP3, (G) Caspase-1 (H) IL-1 beta
When reviewing the results of the cognitive functioning tests the mice performed, the data shows that following the induction of TBI the mice exhibited significantly impaired performance levels in the NOR test, indicating an impairment of short term memory. When Oxiracetam was administered the scores for the novel object C1 all significantly increased. Additionally, the results of the Y-maze test revealed that there were impairments in spatial memory following induction of TBI. This impairment was reduced with administration of Oxiracetam. Finally, following TBI, the time of latency it took for the test subjects to find the hidden platform in the Morris Water Maze was markedly increased, however, following treatment with the nootropic, latency time was found to have significantly decreased [1].

Figure 3: Results of the cognitive function tests including NOR, Y-maze, and Morris Water Maze
2) After performing ligation on the right carotid artery of each test subject, the results of this portion of the study found that the ipsilateral hemisphere of the brain was visibly edematous 3 days after the insult occurred. Treatment with Oxiracetam was found to alleviate the post-insult edema in a dose dependent manner. Additionally, analysis performed through TTC staining found that there was a large infarct core following the hypoxic-ischemic insult that was significantly decreased following treatment with the nootropic. These findings were solidified through the data gathered from Nissl staining of neurons and MRI angiography that emphasized a reduction in cerebral edema and ischemic infarction after doses of 1 mg/kg and 10 mg/kg of Oxiracetam was administered [2].
The research team also completed reflex testing after the hypoxic-ischemic insult took place. In comparison to the control group, the test subjects that underwent the insult with no nootropic treatment experienced increased limb turn over time. However, this time measurement was significantly reduced in the experimental groups that were administered Oxiracetam after the insult occurred. The results of the negative geotaxis reflex had similar findings that mice that underwent insult with no additional treatment took longer to turn 180 degrees and climb to the top of the ramp. Again, the time measurement was drastically decreased in the mice receiving treatment with the nootropic. Finally, the findings derived from the cliff avoidance reflex were in line with the righting reflex and the negative geotaxis reflex where the time to completion increased after insult and was decreased with Oxiracetam administration.
The hypoxic-ischemic insult was also found to elicit an inflammatory response that led to further inflammatory responses and increased apoptosis of cells. That being said, the research team performed a TUNEL assay to detect the total number of apoptotic cells present after the insult occurred. There was a significantly lower amount of cells remaining in the ipsilateral brain hemisphere in the untreated experimental mice. Additionally, this is related to the level of TUNEL-positive cells that was also drastically increased in the mice that suffered from the hypoxic-ischemic insult when compared to the control group. Apoptosis was shown to decrease in a dose dependent manner in the lesioned cerebral cortex following treatment with Oxiracetam [2].
Furthermore, Western Blot analysis was used to observe the expression of cleaved caspase-3. Caspase-3 levels were found to be elevated following the hypoxic-ischemic insult, however, these increased levels were significantly counteracted following treatment with Oxiracetam. The cleaved caspase-3 levels were costained with NeuN and double-immunofluorescence labeled in order to check the level and type of apoptotic neurons present after the insult. The number of these double-positive neurons were higher in the ipsilateral hemispheres of the mice that underwent treatment. The amount of double-positive neurons and total neuron loss was shown to decrease in a dose dependent manner following treatment with Oxiracetam.
As it was previously mentioned, microglia tend to be overactive in the immature brain following a hypoxic-ischemic insult. Western Blot analysis revealed that expression of Iba1 was significantly increased after the insult without treatment with Oxiracetam. In the experimental groups administered varying doses of the nootropic, levels of Iba1 significantly decreased in a dose-dependent manner. Additionally, treatment with Oxiracetam was shown to drastically reduce the area of damage surrounding the infarct core, the penumbra. It is important to note that with a 1 mg/kg dose of the nootropic the penumbra was significantly narrowed, and with a 10 mg/kg dose of the nootropic the penumbra almost completely disappeared [2].
Based on the ameliorating effects that the Oxiracetam had on the penumbra the research team was able to conclude that Oxiracetam plays an important role in salvaging the area surrounding the infarct core and suppressing over-activation of the microglia. Furthermore, LUMINEX assay was used to identify inflammatory cytokines following the hypoxic-ischemic insult. Treatment with the nootropic was shown to decrease levels of IL-1 beta, IL-6, and TNF-alpha. The results of RT-PCR similarly reported the mRNA expression of iNOS and COX-2 decreased with Oxiracetam treatment. In terms of the oxygen-glucose deprivation treatment, the nootropic was found to dose-dependently increase cell viability in the damaged areas [2].

Figure 4: Changes in inflammatory cytokines: A) IL-1 beta, B) IL-6, C) TNF-alpha, D) IL-4, E) IL-10, F) iNOS, G) COX-2; in response to treatment with Oxiracetam.
Disclaimer
**LAB USE ONLY**
*This information is for educational purposes only and does not constitute medical advice. THE PRODUCTS DESCRIBED HEREIN ARE FOR RESEARCH USE ONLY. All clinical research must be conducted with oversight from the appropriate Institutional Review Board (IRB). All preclinical research must be conducted with oversight from the appropriate Institutional Animal Care and Use Committee (IACUC) following the guidelines of the Animal Welfare Act (AWA).
Citations
[1] Youn DH, Han SW, Kim JT, Choi H, Lee A, Kim N, Jung H, Hong EP, Park CH, Lee Y, Cho SM, Jeon JP. Oxiracetam alleviates anti-inflammatory activity and ameliorates cognitive impairment in the early phase of traumatic brain injury. Acta Neurochir (Wien). 2023 Aug;165(8):2201-2210. doi: 10.1007/s00701-023-05674-8. Epub 2023 Jun 29. PMID: 37380907.
[2] Wang D, Wei Y, Tian J, He D, Zhang R, Ji X, Huang X, Sun J, Gao J, Wang Z, Pang Q, Liu Q. Oxiracetam Mediates Neuroprotection Through the Regulation of Microglia Under Hypoxia-Ischemia Neonatal Brain Injury in Mice. Mol Neurobiol. 2021 Aug;58(8):3918-3937. doi: 10.1007/s12035-021-02376-z. Epub 2021 Apr 22. PMID: 33886092.
Oxiracetam is sold for laboratory research use only. Terms of sale apply. Not for human consumption, nor medical, veterinary, or household uses. Please familiarize yourself with our Terms & Conditions prior to ordering.




| File Name | View/Download |
| 01-25-2024-Umbrella-Labs-Oxiracetam-Certificate-Of-Analysis-COA.pdf |
VIEW CERTIFICATES OF ANALYSIS (COA)
Additional information
| Weight | 1 Gram, 5 Grams, 10 Grams |
|---|